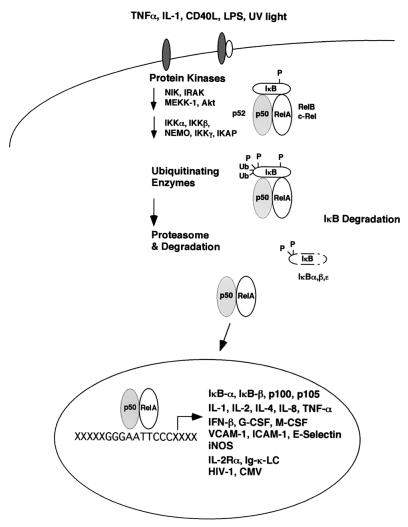
FIG. 2.
In unstimulated cells, the Rel/NF-κB homo- and heterodimers associate with members of the family of inhibitor proteins called IκB and remain as an inactive pool in the cytoplasm. Upon stimulation by different agents like IL-1, TNF-α, CD40L, LPS, or UV light, IκB molecules are rapidly phosphorylated, ubiquitinated, and degraded, allowing the NF-κB dimers to translocate to the nucleus and regulate transcription through binding to κB sites.