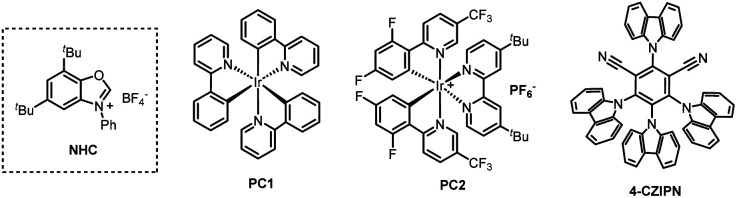
Table 1. Optimization of reaction conditionsa.

| ||
|---|---|---|
| Entry | Variation from the standard conditions | Yieldb |
| 1 | None | 78% |
| 2 | PC1 instead of PC2 | 7% |
| 3 | 4-CZIPN | 71% |
| 4 | Et3N/KOtBu instead of pyridine | Trace |
| 5 | MTBE instead of THF | 34% |
| 6 | DCM instead of MeCN | Trace |
| 7 | DMSO instead of MeCN | 40% |
| 8 | DCE instead of MeCN | 20% |
| 9 | THF instead of MeCN | 71% |
| 10 | DIPEA instead of HCOOCs | N.D. |
| 11 | Cs2CO3 instead of HCOOCs | 42% |
| 12 | In the absence of base | Trace |
| 13 | In the absence of PC | N.D. |
| 14 | In the dark | N.D. |
| ||
Unless otherwise noted, all reactions were performed with 1 (0.25 mmol), 2 (0.10 mmol), NHC (0.23 mmol), pyridine (0.46 mmol), solvent A (1.5 mL), 15 min; then photocatalyst (0.002 mmol), base (0.2 mmol), solvent B (1.0 mL), blue LEDs at rt for 12 h.
Yield determined by 19F NMR using PhCF3 as an internal standard. N.D. = no detection.
