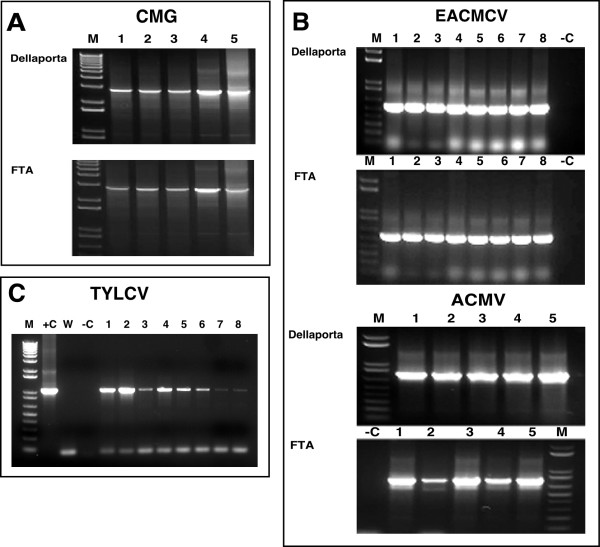
Figure 2.
PCR amplification of geminiviruses components from symptomatic leaves of greenhouse-grown plants using traditional DNA isolation methods and FTA technology. (a) amplification of the 2.8 kb B component of cassava mosaic geminiviruses using universal primers UniF and UniR (Table 1) from independent infected plants. Template DNA was obtained either by extraction and purification of total DNA according to Dellaporta et al. [1] (0.2 μg template) or by elution of viral DNA from leaf tissue pressed onto FTA Classic Cards. (b) amplification of East African cassava Cameroon virus (EACMCV) (lanes 1–8) and African cassava mosaic virus (ACMV) (lanes 1–5) from diseased cassava plants isolated by Dellaporta-based methods (0.2 μg template) or from viral DNA isolated from leaf tissue pressed onto FTA cards. A 550 bp fragment of the B genomic component of EACMCV was amplified using primers EAB555/F and EAB555/R (Table 1) and a 500 bp fragment of the coat protein gene from the A genomic component of ACMV generated using primers JSP001 and JSP002 (Table 1). (c) amplification of the 1.07 kb C1 gene of the Egyptian strain of the monopartite tomato yellow leaf curl virus eluted from infected tomato (lanes 1,2,5 and 6) and N. benthamiana (lanes 3, 4, 7 and 8) leaves pressed onto FTA cards. Increasing the time which paper punches were soaked in elution buffer from 30 minutes (lanes 1–4) to 12 hours (lanes 5–8) increased the signal strength of the amplified viral sequence in both plant species. In all cases, M: marker, +C: positive control, -C: negative control, W: water control. CMG – cassava mosaic geminiviruses; ACMV – African cassava mosaic virus; EACMV – East African cassava mosaic virus; TYLCV – Tomato yellow leaf curl virus.