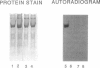
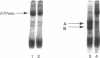Abstract
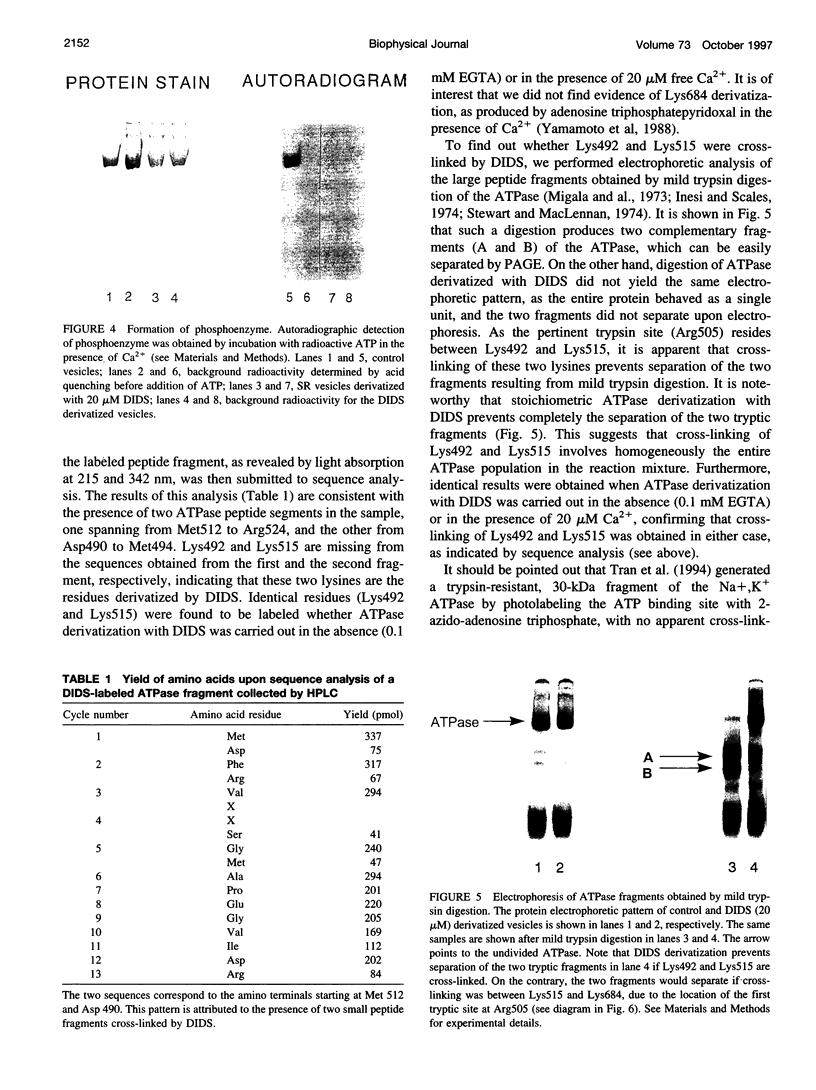
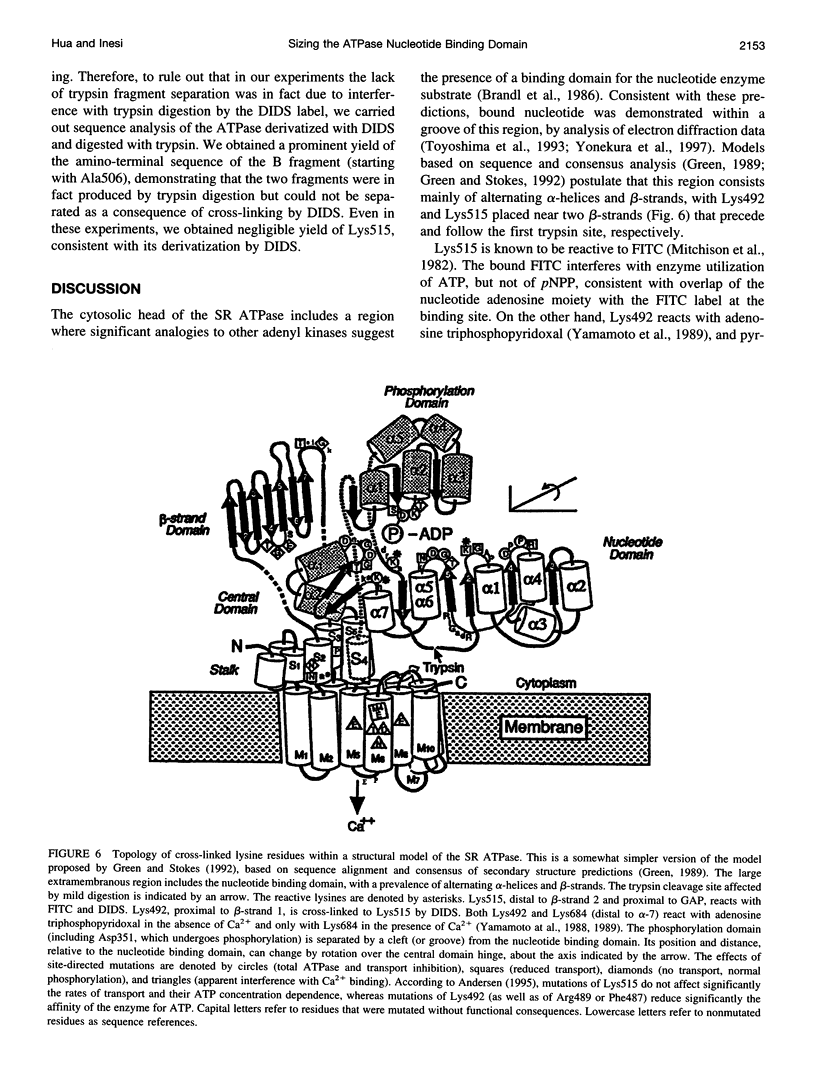
Sarcoplasmic reticulum (SR) Ca2+ ATPase was derivatized with 4,4'-diisothiocyanatostilbene-2,2'-sulfonic acid (DIDS), and complete enzyme inactivation was produced with a molecular stoichiometry of one DIDS per ATPase. It was determined by peptide analysis and sequencing that Lys492 and Lys515 were the ATPase residues derivatized by DIDS. Lack of electrophoretic resolution of the two peptide fragments that result from a single tryptic cut at Arg505 demonstrated that the two derivatized residues were cross-linked. Cross-linking of Lys492 and Lys515 by DIDS interfered with ATPase utilization of both ATP and p-nitrophenyl phosphate substrates, whereas derivatization of only Lys515 with fluorescein isothiocyanate interfered with ATPase utilization of ATP but not of p-nitrophenyl phosphate. Cross-linking with DIDS implies a distance of approximately 13 A between Lys492 and Lys515, which corresponds to the length of ATP bound in an extended configuration. Therefore, within the groove of the nucleotide binding domain, the ATP substrate is positioned with the adenosine moiety near Lys515 and its terminal phosphate near Lys492.
Full text
PDF
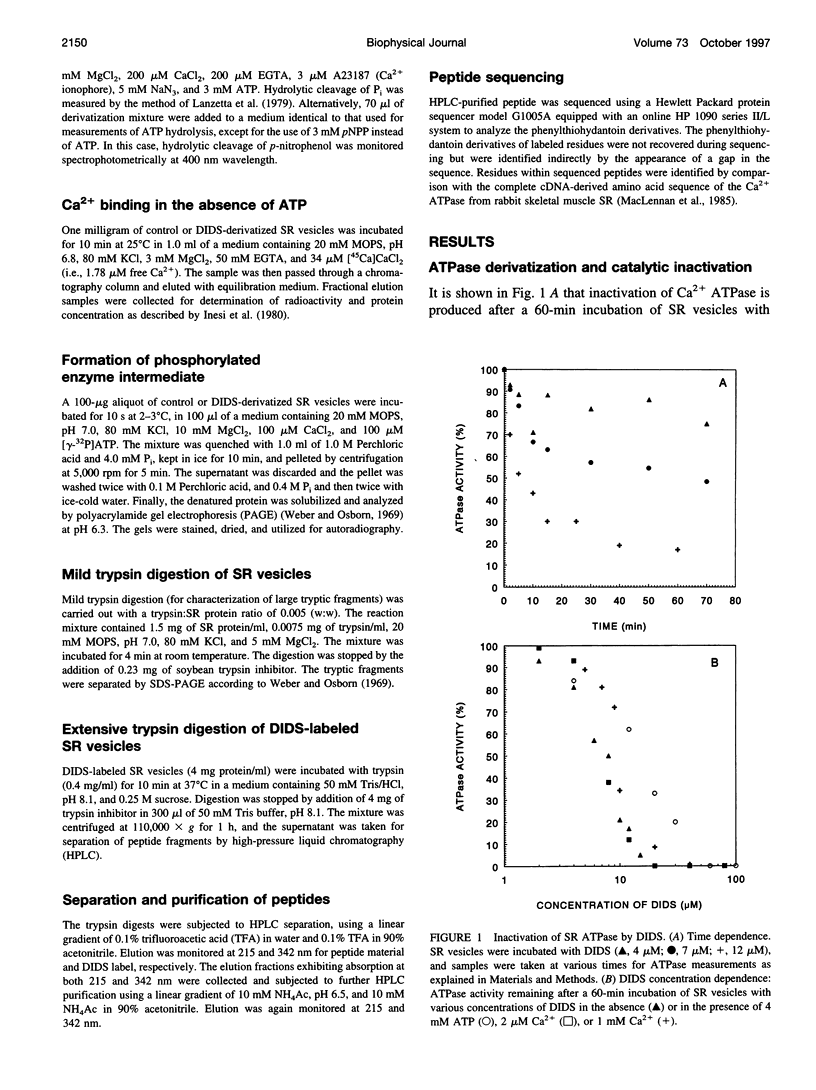
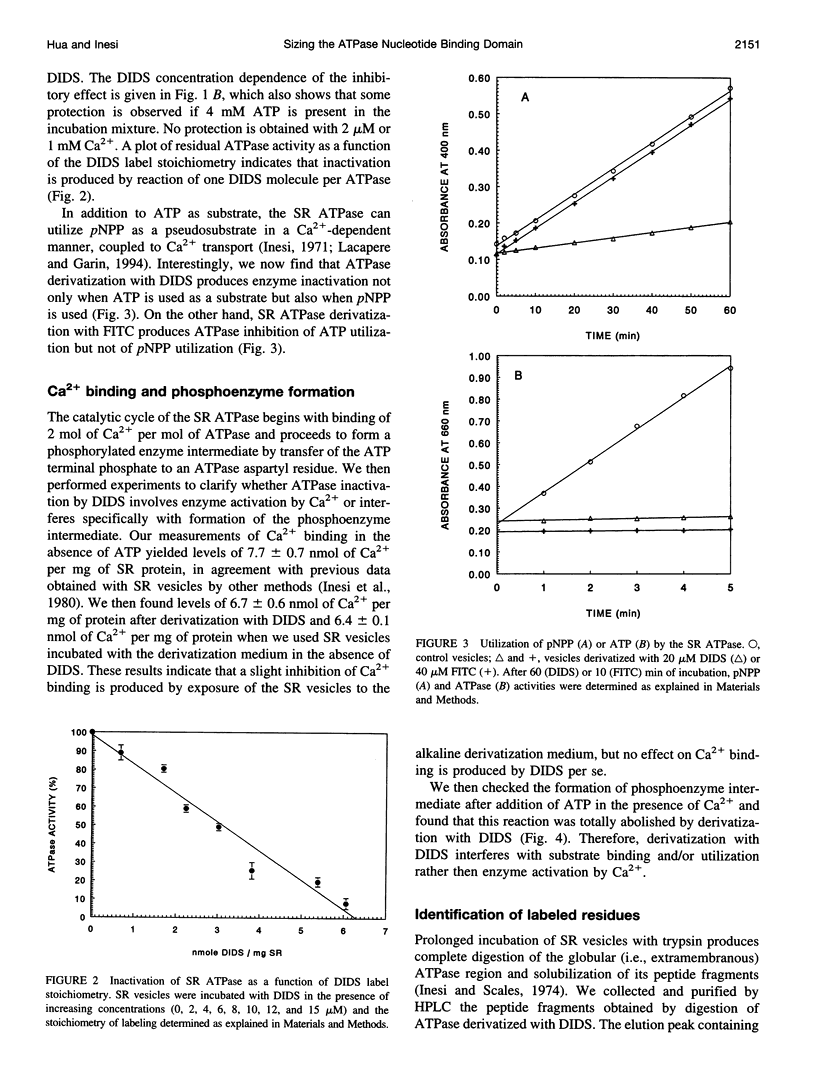


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. P. Dissection of the functional domains of the sarcoplasmic reticulum Ca(2+)-ATPase by site-directed mutagenesis. Biosci Rep. 1995 Oct;15(5):243–261. doi: 10.1007/BF01788358. [DOI] [PubMed] [Google Scholar]
- Andersen J. P., Møller J. V., Jørgensen P. L. The functional unit of sarcoplasmic reticulum Ca2+-ATPase. Active site titration and fluorescence measurements. J Biol Chem. 1982 Jul 25;257(14):8300–8307. [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M., Mitchinson C., Green N. M. 1H-NMR studies on nucleotide binding to the sarcoplasmic reticulum Ca2+ ATPase. Determination of the conformations of bound nucleotides by the measurement of proton-proton transferred nuclear Overhauser enhancements. Eur J Biochem. 1982 Nov;128(1):113–117. [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Green N. M. ATP-driven cation pumps: alignment of sequences. Biochem Soc Trans. 1989 Dec;17(6):972–972. doi: 10.1042/bst0170972. [DOI] [PubMed] [Google Scholar]
- Green N. M., Stokes D. L. Structural modelling of P-type ion pumps. Acta Physiol Scand Suppl. 1992;607:59–68. [PubMed] [Google Scholar]
- Inesi G., Cantilina T., Yu X., Nikic D., Sagara Y., Kirtley M. E. Long-range intramolecular linked functions in activation and inhibition of SERCA ATPases. Ann N Y Acad Sci. 1992 Nov 30;671:32–48. doi: 10.1111/j.1749-6632.1992.tb43782.x. [DOI] [PubMed] [Google Scholar]
- Inesi G., Kurzmack M., Coan C., Lewis D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Apr 10;255(7):3025–3031. [PubMed] [Google Scholar]
- Inesi G., Scales D. Tryptic cleavage of sarcoplasmic reticulum protein. Biochemistry. 1974 Jul 30;13(16):3298–3306. doi: 10.1021/bi00713a019. [DOI] [PubMed] [Google Scholar]
- Inesi G. p-nitrophenyl phosphate hydrolysis and calcium ion transport in fragmented sarcoplasmic reticulum. Science. 1971 Mar 5;171(3974):901–903. doi: 10.1126/science.171.3974.901. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacapère J. J., Garin J. Interaction of 4-azido-2-nitrophenyl phosphate, a pseudosubstrate, with the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1994 Mar 8;33(9):2586–2593. doi: 10.1021/bi00175a030. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- McIntosh D. B. Glutaraldehyde cross-links Lys-492 and Arg-678 at the active site of sarcoplasmic reticulum Ca(2+)-ATPase. J Biol Chem. 1992 Nov 5;267(31):22328–22335. [PubMed] [Google Scholar]
- McIntosh D. B., Woolley D. G., Berman M. C. 2',3'-O-(2,4,6-trinitrophenyl)-8-azido-AMP and -ATP photolabel Lys-492 at the active site of sarcoplasmic reticulum Ca(2+)-ATPase. J Biol Chem. 1992 Mar 15;267(8):5301–5309. [PubMed] [Google Scholar]
- Migala A., Agostini B., Hasselbach W. Tryptic fragmentation of the calcium transport system in the sarcoplasmic reticulum. Z Naturforsch C. 1973 Mar-Apr;28(3):178–182. doi: 10.1515/znc-1973-3-414. [DOI] [PubMed] [Google Scholar]
- Mitchinson C., Wilderspin A. F., Trinnaman B. J., Green N. M. Identification of a labelled peptide after stoicheiometric reaction of fluorescein isothiocyanate with the Ca2+ -dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 1982 Sep 6;146(1):87–92. doi: 10.1016/0014-5793(82)80710-2. [DOI] [PubMed] [Google Scholar]
- Okubo K., Kang D., Hamasaki N., Jennings M. L. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4'-diisothiocyanodihydrostilbene-2,2'-disulfonic acid) molecule. J Biol Chem. 1994 Jan 21;269(3):1918–1926. [PubMed] [Google Scholar]
- Pedemonte C. H., Kaplan J. H. Inhibition and derivatization of the renal Na,K-ATPase by dihydro-4,4'-diisothiocyanatostilbene-2,2'-disulfonate. Biochemistry. 1988 Oct 4;27(20):7966–7973. doi: 10.1021/bi00420a056. [DOI] [PubMed] [Google Scholar]
- Pick U., Bassilian S. Modification of the ATP binding site of the Ca2+ -ATPase from sarcoplasmic reticulum by fluorescein isothiocyanate. FEBS Lett. 1981 Jan 12;123(1):127–130. doi: 10.1016/0014-5793(81)80035-x. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Regulation of the conformation transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J Biol Chem. 1982 Jun 10;257(11):6120–6126. [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Surface particles of sarcoplasmic reticulum membranes. Structural features of the adenosine triphosphatase. J Biol Chem. 1974 Feb 10;249(3):985–993. [PubMed] [Google Scholar]
- Toyoshima C., Sasabe H., Stokes D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature. 1993 Apr 1;362(6419):467–471. doi: 10.1038/362469a0. [DOI] [PubMed] [Google Scholar]
- Tran C. M., Huston E. E., Farley R. A. Photochemical labeling and inhibition of Na,K-ATPase by 2-Azido-ATP. Identification of an amino acid located within the ATP binding site. J Biol Chem. 1994 Mar 4;269(9):6558–6565. [PubMed] [Google Scholar]
- Watanabe T., Inesi G. The use of 2',3'-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate for studies of nucleotide interaction with sarcoplasmic reticulum vesicles. J Biol Chem. 1982 Oct 10;257(19):11510–11516. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamagata K., Daiho T., Kanazawa T. Labeling of lysine 492 with pyridoxal 5'-phosphate in the sarcoplasmic reticulum Ca(2+)-ATPase. Lysine 492 residue is located outside the fluorescein 5-isothiocyanate-binding region in or near the ATP binding site. J Biol Chem. 1993 Oct 5;268(28):20930–20936. [PubMed] [Google Scholar]
- Yamamoto H., Imamura Y., Tagaya M., Fukui T., Kawakita M. Ca2(+)-dependent conformational change of the ATP-binding site of Ca2(+)-transporting ATPase of sarcoplasmic reticulum as revealed by an alteration of the target-site specificity of adenosine triphosphopyridoxal. J Biochem. 1989 Dec;106(6):1121–1125. doi: 10.1093/oxfordjournals.jbchem.a122976. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Tagaya M., Fukui T., Kawakita M. Affinity labeling of the ATP-binding site of Ca2+-transporting ATPase of sarcoplasmic reticulum by adenosine triphosphopyridoxal: identification of the reactive lysyl residue. J Biochem. 1988 Mar;103(3):452–457. doi: 10.1093/oxfordjournals.jbchem.a122291. [DOI] [PubMed] [Google Scholar]
- Yonekura K., Stokes D. L., Sasabe H., Toyoshima C. The ATP-binding site of Ca(2+)-ATPase revealed by electron image analysis. Biophys J. 1997 Mar;72(3):997–1005. doi: 10.1016/S0006-3495(97)78752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]