Abstract
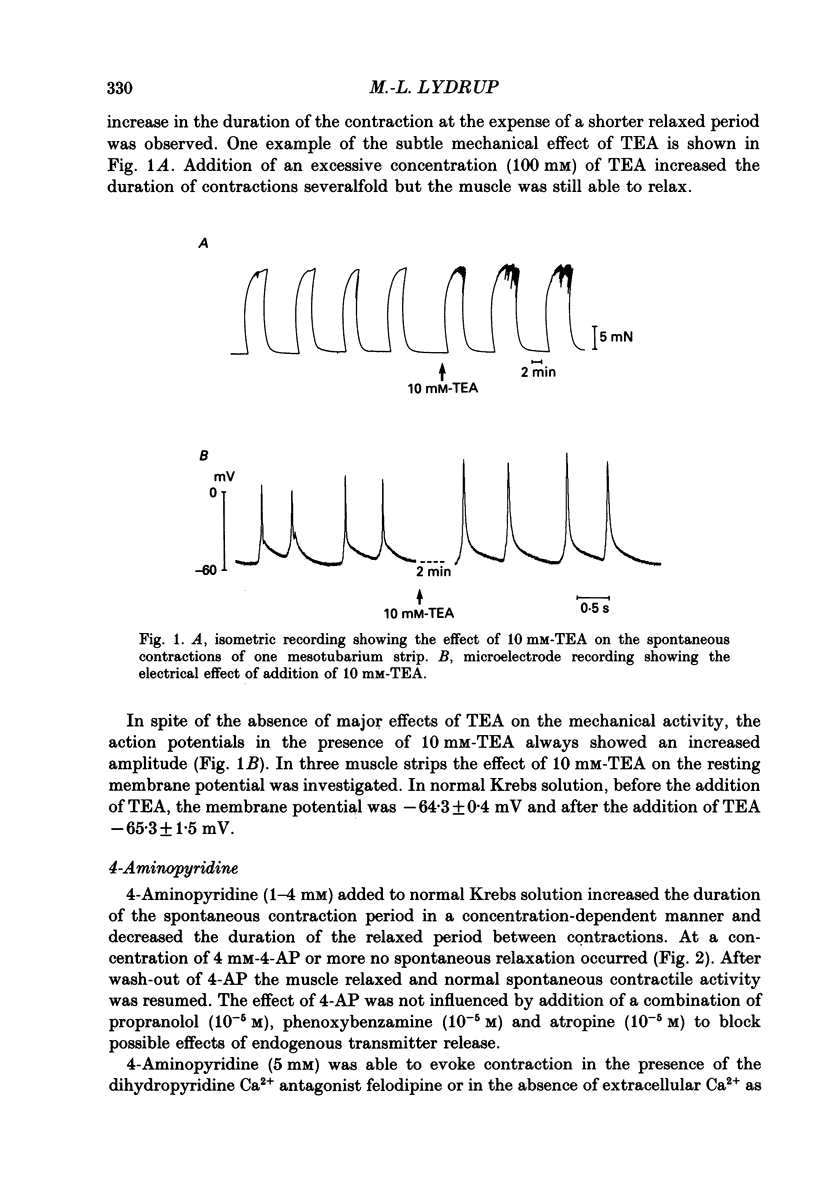
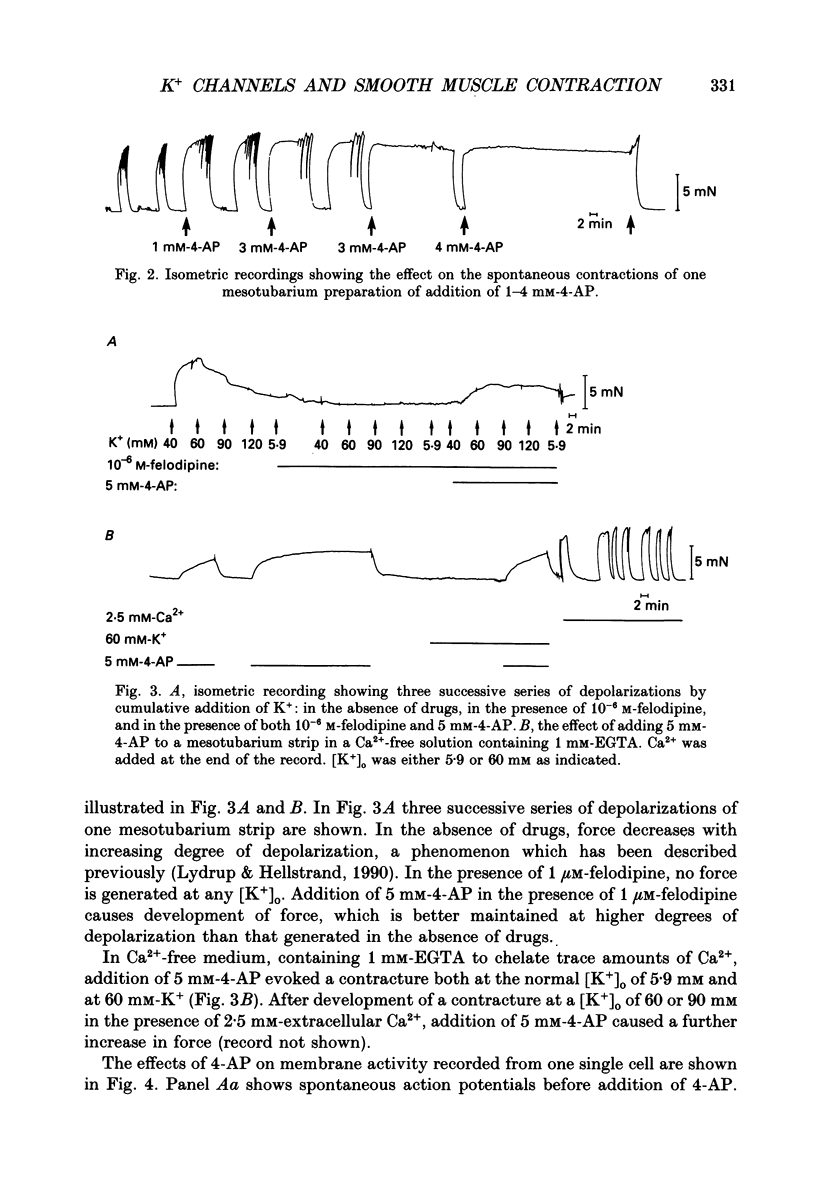
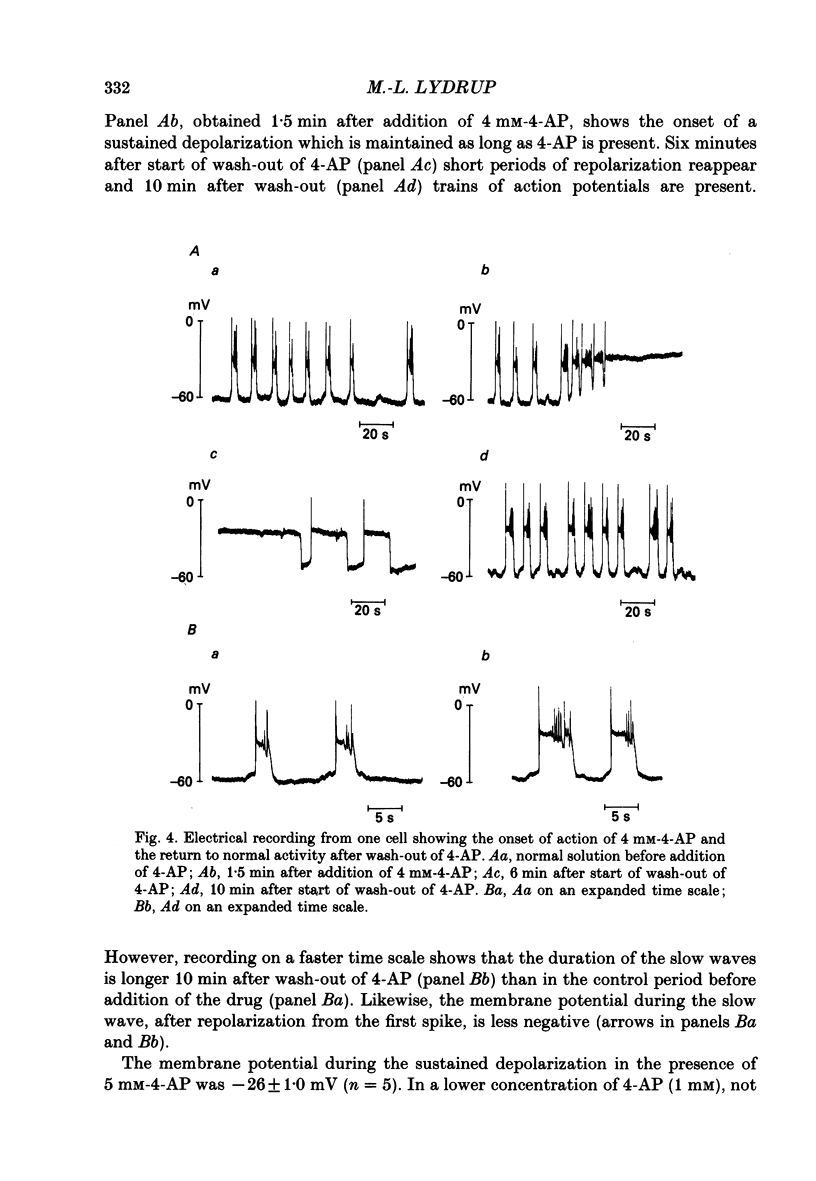
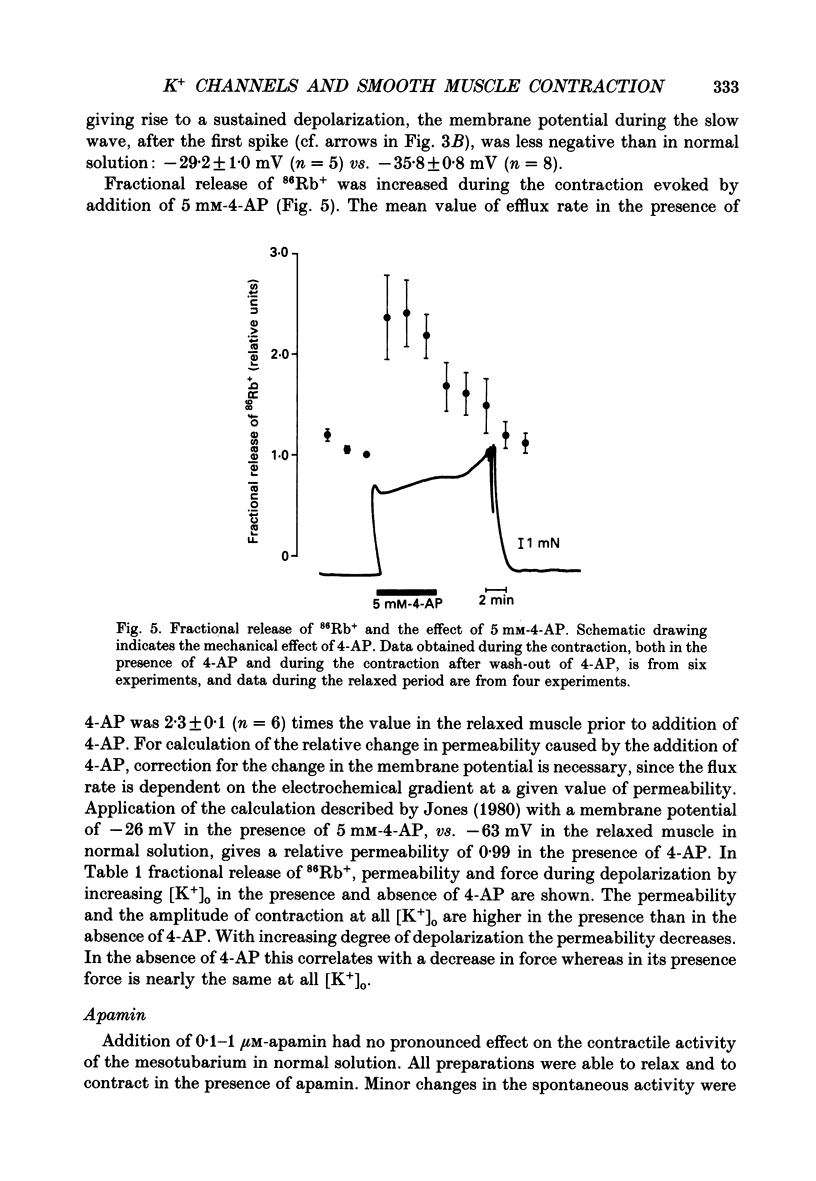
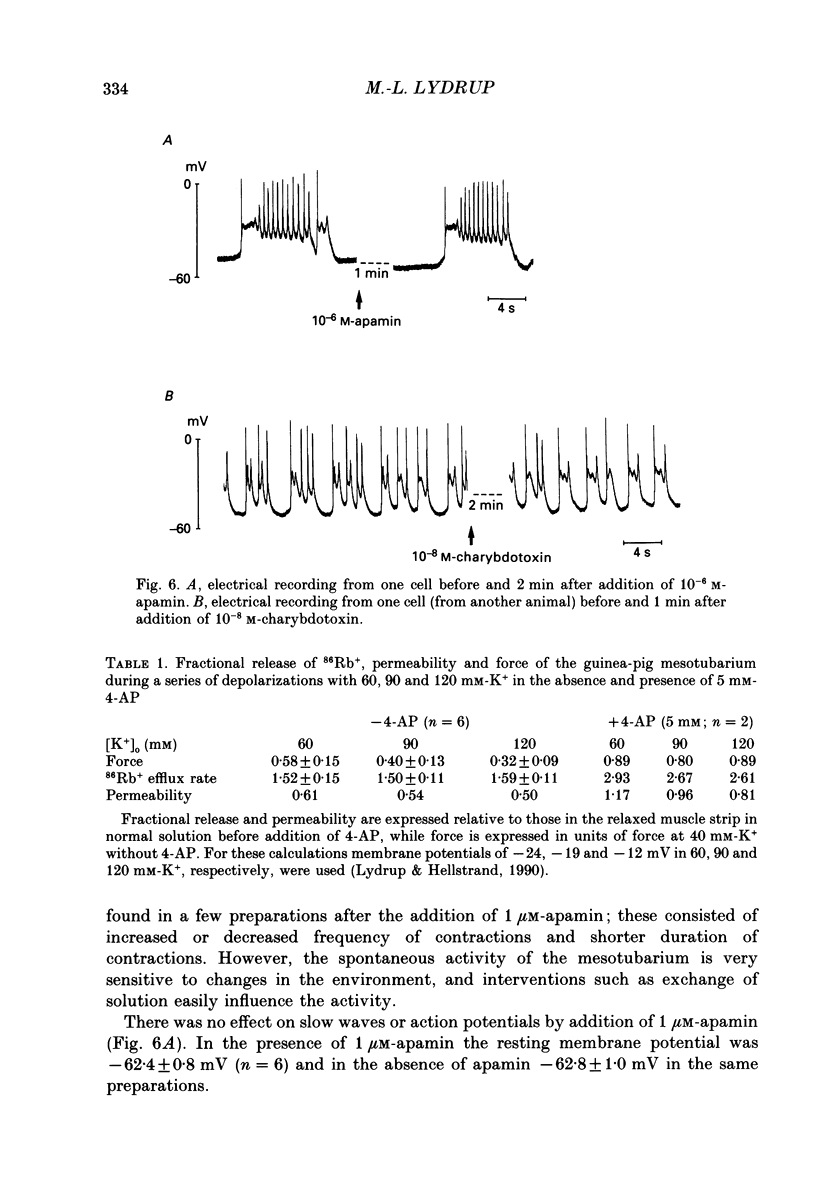
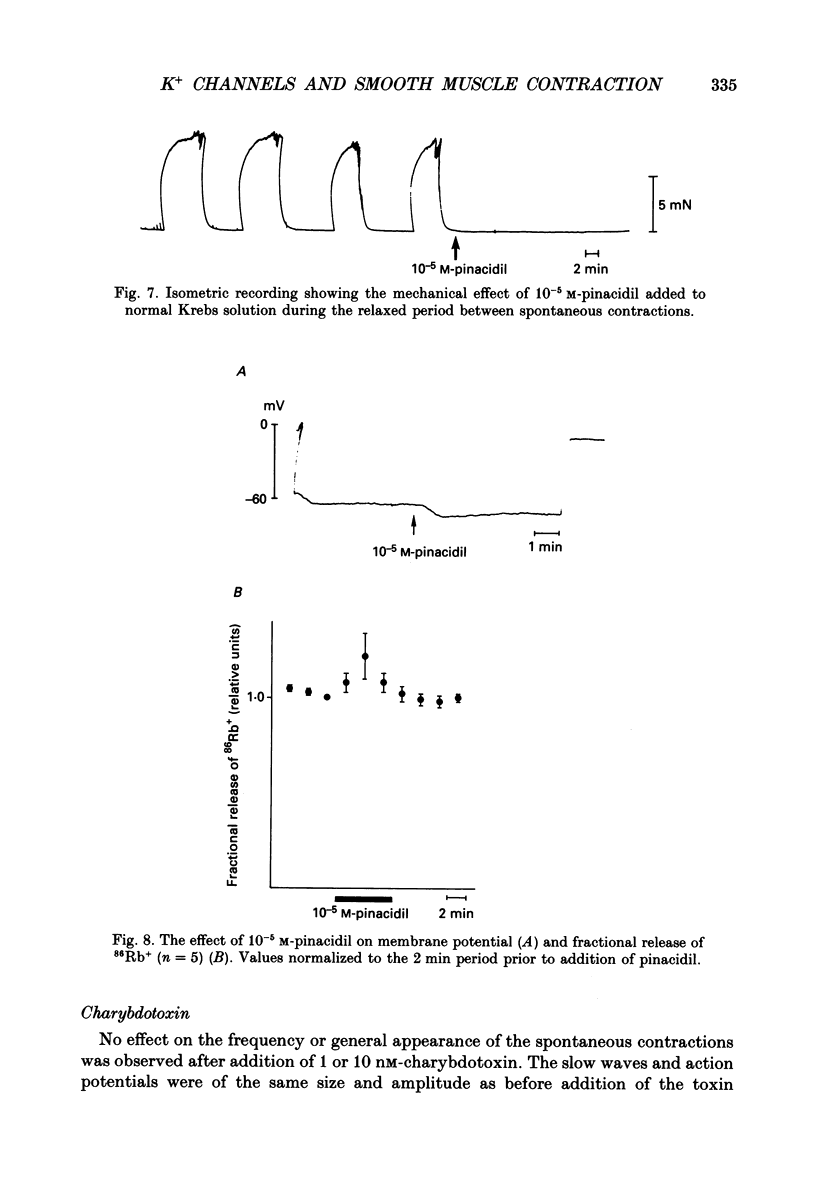
1. The spontaneous electrical and mechanical activity and the efflux rate of 86Rb+ in the guinea-pig mesotubarium were studied in the presence of agents interacting with K+ channels. 2. Tetraethylammonium (TEA, 10 mM) increased the amplitude of the action potentials while having no consistent effect on the frequency or amplitude of spontaneous contractions. 3. 4-Aminopyridine (4-AP, 1-5 mM) caused a graded increase in the duration of the contractions and of the electrical slow waves, and a decrease in the duration of the relaxed period between contractions. At 4 mM-4-AP or more the cell was unable to repolarize from the slow wave and the membrane depolarized to -26 mV from the normal resting potential of -63 mV. The rate of 86Rb+ efflux in the presence of 5 mM-4-AP was higher than that at 60 mM-K+, where the membrane potential is -24 mV. 4. 4-AP (5 mM) evoked a contracture in Ca(2+)-free solution, containing 1 mM-EGTA, both at the normal [K+]o of 5.9 mM and at 60 mM-K+, suggesting release of intracellular Ca2+. 5. Apamin (0.1-1 microM) and charybdotoxin (1-10 nM), blockers of Ca(2+)-dependent K+ channels, were without effects on the spontaneous electrical and mechanical activity. 6. The K+ channel opener pinacidil (10 microM) inhibited the spontaneous contractions and hyperpolarized the membrane by about 7 mV. The permeability to 86Rb+ was increased by a factor of 1.4. 7. It is concluded that different K+ channels are involved in the generation of spikes and slow waves: one sensitive to TEA and responsible for repolarization of the individual action potential, and another sensitive to 4-AP and responsible for repolarization of the slow wave. The duration of the relaxed period can be influenced by activation of K+ channels sensitive to pinacidil.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Slow repetitive activity from fast conductance changes in neurons. Fed Proc. 1978 Jun;37(8):2139–2145. [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Le Guern S., Courteix J., Laville M., Cavero I. Potassium channel openers act through an activation of ATP-sensitive K+ channels in guinea-pig cardiac myocytes. Pflugers Arch. 1989 Sep;414(6):669–675. doi: 10.1007/BF00582134. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand P., Lydrup M. L. Spontaneous electrical and contractile activity correlated to 86Rb+ efflux in smooth muscle of guinea-pig mesotubarium. J Physiol. 1988 Dec;407:587–597. doi: 10.1113/jphysiol.1988.sp017433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M., Amédée T., Edwards D., Mironneau J., Savineau J. P., Small R. C., Weston A. H. The relaxant action of BRL 34915 in rat uterus. Br J Pharmacol. 1987 Aug;91(4):803–813. doi: 10.1111/j.1476-5381.1987.tb11279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. Effect of 4-aminopyridine on potassium permeability of canine tracheal smooth muscle cell membrane. Jpn J Pharmacol. 1983 Feb;33(1):201–208. doi: 10.1254/jjp.33.201. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander S., Arner A., Johansson B. Effects of 4-aminopyridine on mechanical activity and noradrenaline release in the rat portal vein in vitro. Eur J Pharmacol. 1977 Dec 15;46(4):351–361. doi: 10.1016/0014-2999(77)90229-1. [DOI] [PubMed] [Google Scholar]
- Lydrup M. L., Hellstrand P. Effects of extracellular K+ and Ca2+ on membrane potential, contraction and 86Rb+ efflux in guinea-pig mesotubarium. Pflugers Arch. 1990 Mar;415(6):664–670. doi: 10.1007/BF02584003. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P. Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. J Physiol. 1980 May;302:411–425. doi: 10.1113/jphysiol.1980.sp013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., INOMATA H. Effect of tetraethyl ammonium ion on the electrical activity of smooth muscle cell. Nature. 1963 Mar 2;197:908–909. doi: 10.1038/197908a0. [DOI] [PubMed] [Google Scholar]
- Savage A. O. A comparison of the actions of 4-aminopyridine, caffeine and quinine on the toad isolated rectus abdominis muscle. Comp Biochem Physiol C. 1989;92(1):27–33. doi: 10.1016/0742-8413(89)90197-7. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]


