Abstract
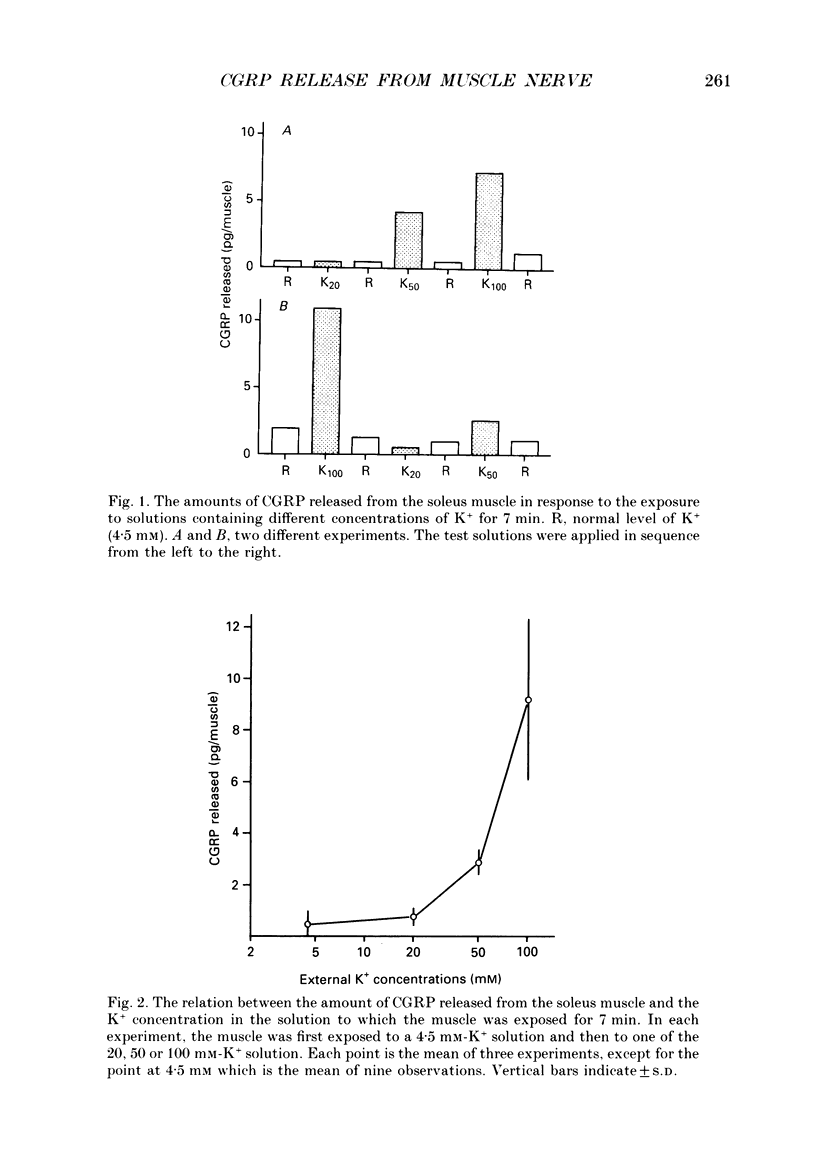
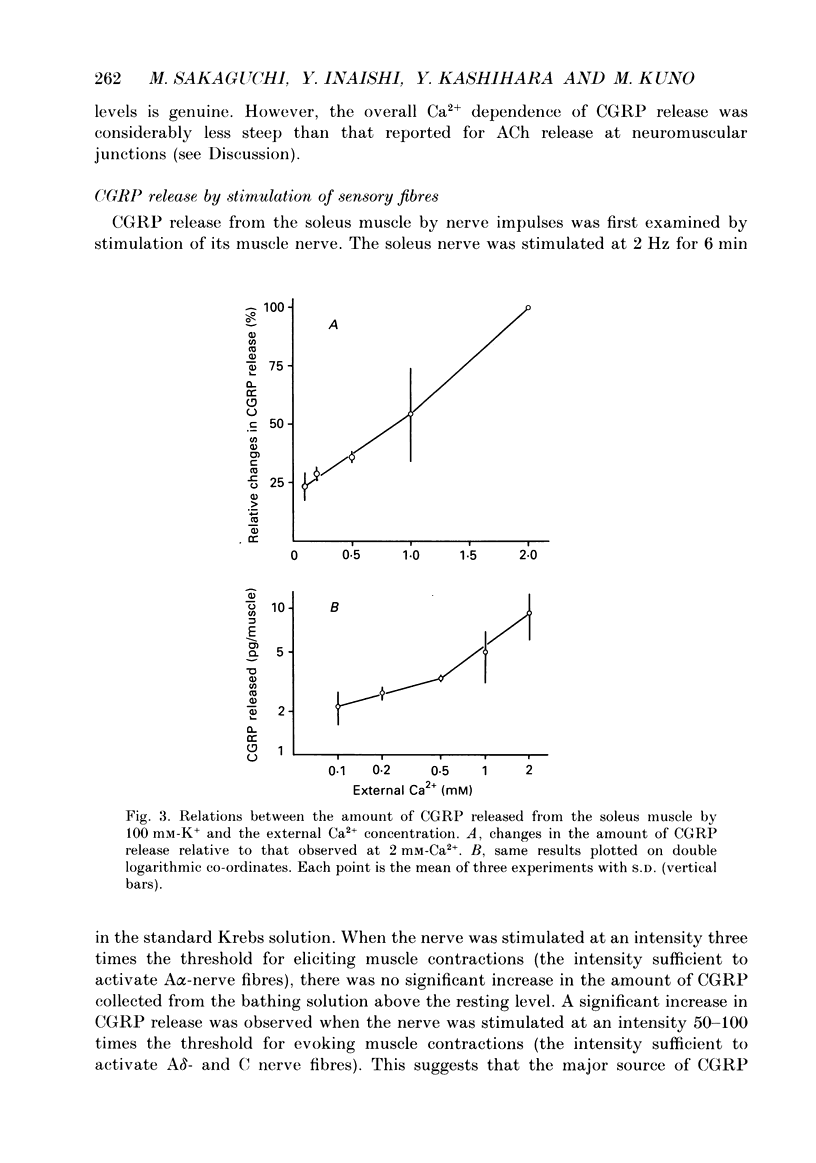
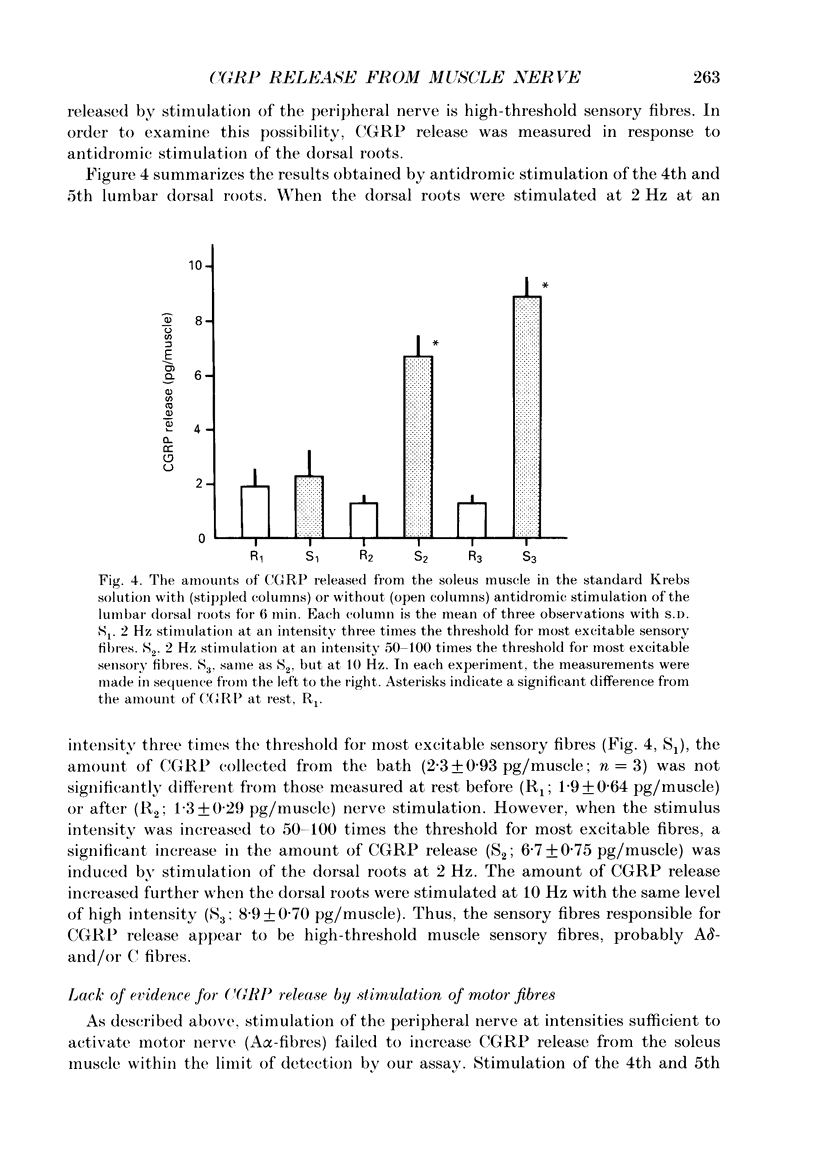
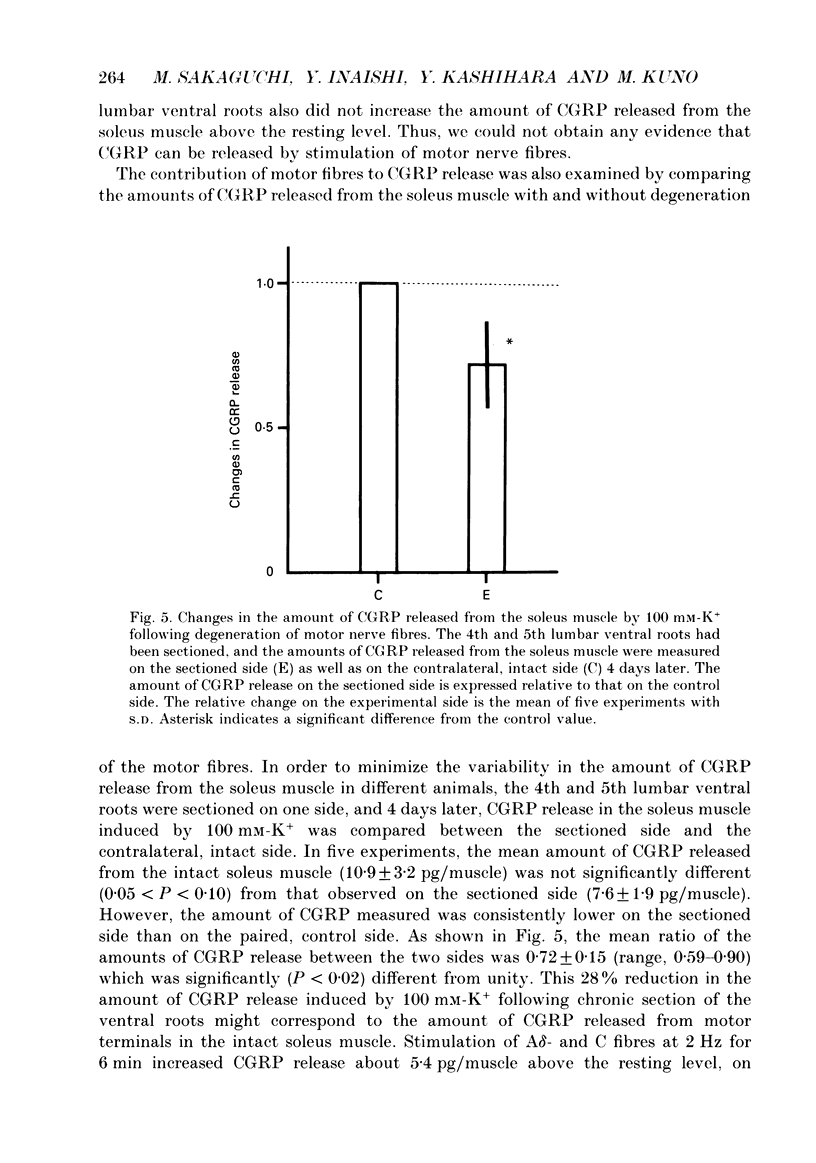
1. The amount of calcitonin gene-related peptide (CGRP) released from the isolated rat soleus muscle was measured by enzyme immunoassay. 2. When the soleus muscle was exposed to a solution containing high K+ (20-100 mM) in the presence of tetrodotoxin, the amount of CGRP released into the bathing medium increased with an increase in the K+ concentration. 3. The exposure to 100 mM-K+ did not increase CGRP release from chronically denervated soleus muscles or from pieces of the soleus nerve separated from the muscle. 4. The amount of CGRP released from the isolated muscle by 100 mM-K+ depended on the external Ca2+ concentration. The slope of the relation between the amount of CGRP release and the Ca2+ concentration was less than one on double logarithmic co-ordinates. 5. Following chronic section of the lumbar ventral roots, the mean amount of CGRP released from the soleus muscle by 100 mM-K+ was reduced by 28%, compared with that observed in normal muscle. 6. Antidromic stimulation of the lumbar dorsal roots at an intensity three times the threshold for most excitable sensory fibres failed to induce CGRP release from the soleus muscle, whereas stimulation at intensities 50-100 times the threshold increased significantly the amount of CGRP release from the muscle. 7. Stimulation of the muscle nerve at an intensity sufficient to activate the alpha-motor fibres did not release CGRP from the soleus muscle or from the diaphragm. 8. It is concluded that the major source of CGRP released from skeletal muscle is A delta- and/or C sensory terminals and that the Ca2+ dependence of CGRP release is less steep than that reported for acetylcholine release at neuromuscular junctions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agoston D. V., Conlon J. M., Whittaker V. P. Selective depletion of the acetylcholine and vasoactive intestinal polypeptide of the guinea-pig myenteric plexus by differential mobilization of distinct transmitter pools. Exp Brain Res. 1988;72(3):535–542. doi: 10.1007/BF00250599. [DOI] [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Guerra F. J., Zaidi M., Bevis P., MacIntyre I., Emson P. C. Evidence for release of calcitonin gene-related peptide and neurokinin A from sensory nerve endings in vivo. Neuroscience. 1988 Jun;25(3):839–846. doi: 10.1016/0306-4522(88)90039-5. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci Lett. 1986 Oct 30;71(1):59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Henke H., Lundberg J. M., Petermann J. B., Hökfelt T., Fischer J. A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987 Mar-Apr;8(2):399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Fried G., Franck J., Brodin E., Born W., Fischer J. A., Hiort W., Hökfelt T. Evidence for differential storage of calcitonin gene-related peptide, substance P and serotonin in synaptosomal vesicles of rat spinal cord. Brain Res. 1989 Oct 16;499(2):315–324. doi: 10.1016/0006-8993(89)90780-4. [DOI] [PubMed] [Google Scholar]
- Gibson S. J., Polak J. M., Bloom S. R., Sabate I. M., Mulderry P. M., Ghatei M. A., McGregor G. P., Morrison J. F., Kelly J. S., Evans R. M. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci. 1984 Dec;4(12):3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq C. B., Hulsebosch C. E., Coggeshall R. E., Perez-Polo J. R. The effects of nerve growth factor and its antibodies on axonal numbers in the medial gastrocnemius nerve of the rat. Brain Res. 1984 May 7;299(1):9–14. doi: 10.1016/0006-8993(84)90782-0. [DOI] [PubMed] [Google Scholar]
- Kashihara Y., Sakaguchi M., Kuno M. Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. J Neurosci. 1989 Nov;9(11):3796–3802. doi: 10.1523/JNEUROSCI.09-11-03796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger L., Kumazawa T., Mizumura K., Sato J., Yeh Y. Observations on electrophysiologically characterized receptive fields of thin testicular afferent axons: a preliminary note on the analysis of fine structural specializations of polymodal receptors. Somatosens Res. 1988;5(4):373–380. doi: 10.3109/07367228809144637. [DOI] [PubMed] [Google Scholar]
- Kuno M., Takahashi T. Effects of calcium and magnesium on transmitter release at Ia synapses of rat spinal motoneurones in vitro. J Physiol. 1986 Jul;376:543–553. doi: 10.1113/jphysiol.1986.sp016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S. M., Jamieson A., Russell N. J., Dockray G. J. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience. 1989;32(3):581–586. doi: 10.1016/0306-4522(89)90281-9. [DOI] [PubMed] [Google Scholar]
- Mason R. T., Peterfreund R. A., Sawchenko P. E., Corrigan A. Z., Rivier J. E., Vale W. W. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature. 1984 Apr 12;308(5960):653–655. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Haimann C., Torri-Tarelli F., Polak J. M., Ceccarelli B., De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy P. W., Lawson S. N. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34(3):623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- McNeill D. L., Coggeshall R. E., Carlton S. M. A light and electron microscopic study of calcitonin gene-related peptide in the spinal cord of the rat. Exp Neurol. 1988 Mar;99(3):699–708. doi: 10.1016/0014-4886(88)90186-0. [DOI] [PubMed] [Google Scholar]
- Molander C., Ygge J., Dalsgaard C. J. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett. 1987 Feb 10;74(1):37–42. doi: 10.1016/0304-3940(87)90047-4. [DOI] [PubMed] [Google Scholar]
- Morton C. R., Hutchison W. D. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31(3):807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- Mulle C., Benoit P., Pinset C., Roa M., Changeux J. P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New H. V., Mudge A. W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. 1986 Oct 30-Nov 5Nature. 323(6091):809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- O'Brien C., Woolf C. J., Fitzgerald M., Lindsay R. M., Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32(2):493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Ohlén A., Lindbom L., Staines W., Hökfelt T., Cuello A. C., Fischer J. A., Hedqvist P. Substance P and calcitonin gene-related peptide: immunohistochemical localisation and microvascular effects in rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):87–93. doi: 10.1007/BF00177756. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Saria A., Gamse R., Petermann J., Fischer J. A., Theodorsson-Norheim E., Lundberg J. M. Simultaneous release of several tachykinins and calcitonin gene-related peptide from rat spinal cord slices. Neurosci Lett. 1986 Jan 30;63(3):310–314. doi: 10.1016/0304-3940(86)90376-9. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986 May;215(1):71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- Takami K., Kawai Y., Shiosaka S., Lee Y., Girgis S., Hillyard C. J., MacIntyre I., Emson P. C., Tohyama M. Immunohistochemical evidence for the coexistence of calcitonin gene-related peptide- and choline acetyltransferase-like immunoreactivity in neurons of the rat hypoglossal, facial and ambiguus nuclei. Brain Res. 1985 Mar 4;328(2):386–389. doi: 10.1016/0006-8993(85)91055-8. [DOI] [PubMed] [Google Scholar]
- Takami K., Kawai Y., Uchida S., Tohyama M., Shiotani Y., Yoshida H., Emson P. C., Girgis S., Hillyard C. J., MacIntyre I. Effect of calcitonin gene-related peptide on contraction of striated muscle in the mouse. Neurosci Lett. 1985 Sep 30;60(2):227–230. doi: 10.1016/0304-3940(85)90248-4. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T., Kuno M. Calcitonin gene-related peptide prevents disuse-induced sprouting of rat motor nerve terminals. J Neurosci. 1988 Oct;8(10):3951–3957. doi: 10.1523/JNEUROSCI.08-10-03951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Yamamoto H., Iio S., Matsumoto N., Wang X. B., Yonehara N., Imai Y., Inoki R., Yoshida H. Release of calcitonin gene-related peptide-like immunoreactive substance from neuromuscular junction by nerve excitation and its action on striated muscle. J Neurochem. 1990 Mar;54(3):1000–1003. doi: 10.1111/j.1471-4159.1990.tb02349.x. [DOI] [PubMed] [Google Scholar]
- White D. M., Leah J. D., Zimmermann M. The localization and release of substance P and calcitonin gene-related peptide at nerve fibre endings in rat cutaneous nerve neuroma. Brain Res. 1989 Dec 4;503(2):198–204. doi: 10.1016/0006-8993(89)91664-8. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Hökfelt T., Lundberg J. M., Forssmann W. G., Reinecke M., Tschopp F. A., Fischer J. A. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett. 1984 Nov 23;52(1-2):199–204. doi: 10.1016/0304-3940(84)90374-4. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Bevis P. J., Girgis S. I., Lynch C., Stevenson J. C., MacIntyre I. Circulating CGRP comes from the perivascular nerves. Eur J Pharmacol. 1985 Nov 5;117(2):283–284. doi: 10.1016/0014-2999(85)90616-8. [DOI] [PubMed] [Google Scholar]


