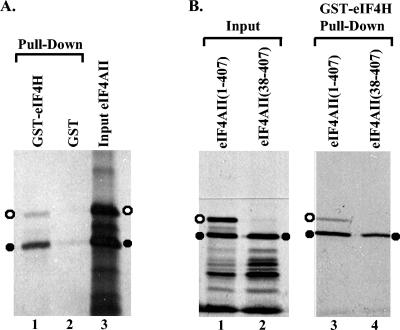
FIG. 1.
Binding of eIF4AII to a GST-eIF4H fusion protein. (A) GST or a GST-eIF4H fusion protein was produced in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing the [35S]methionine-labeled in vitro translation products of full-length eIF4AII mRNA. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography. Lane 1 contains proteins that bound to GST-eIF4H, lane 2 contains material that bound to GST, and lane 3 contains an aliquot of the starting material. The 45-kDa and 41-kDa products of in vitro translation of full-length eIF4AII mRNA are designated by the open and filled circles, respectively. (B) GST or GST-eIF4H was incubated with the in vitro translation products of full-length eIF4AII mRNA (lane 3) or an mRNA encoding only amino acids 38 through 407 of eIF4AII (lane 4). Bound proteins were eluted and analyzed as described in the legend to panel A. Lanes 1 and 2 contain aliquots of the starting material. The 45-kDa and 41-kDa eIF4AII polypeptides are designated by open and filled circles, respectively.