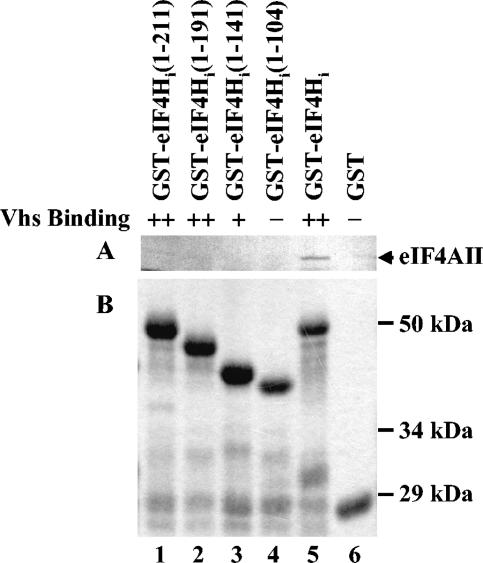
FIG. 10.
eIF4AII binding by deletion mutants of eIF4Hi. Deletion mutants of eIF4Hi were previously used to define the regions of eIF4Hi necessary to bind Vhs by the conventional yeast two-hybrid system and in GST pull-down experiments (18). These mutants were now used to examine the region of eIF4Hi required to bind full-length eIF4AII. Full-length wild-type [35S]methionine-labeled eIF4AII was produced by in vitro translation and analyzed for the ability to bind various fusion proteins containing GST fused to full-length eIF4Hi (lane 5) or deletion mutant forms of eIF4Hi (lanes 1 to 4). Full-length eIF4AII bound by the various GST-eIF4Hi polypeptides and eluted with 10 mM glutathione is shown in the autoradiogram in panel A. The material that bound to just GST is shown in lane 6. The eIF4Hi amino acids that were present in the wild-type and various mutant forms of eIF4Hi are shown in parentheses at the top of each lane of the gel. A Coomassie-stained gel of the mutant and wild-type GST-eIF4Hi proteins used in the GST pull-down reactions is shown in panel B. The ability of the eIF4Hi polypeptides to bind Vhs was reported previously (18) and is summarized above panel A. ++, the mutant eIF4Hi polypeptide binds Vhs as well as wild-type eIF4Hi by both the yeast two-hybrid and GST pull-down assays; +, the mutant eIF4Hi polypeptide binds Vhs as well as wild-type eIF4Hi by the yeast two-hybrid assay but binds threefold-less Vhs in GST pull-down experiments; −, there was no detectable interaction between the mutant eIF4Hi and Vhs by either the yeast two-hybrid or GST pull-down assay.