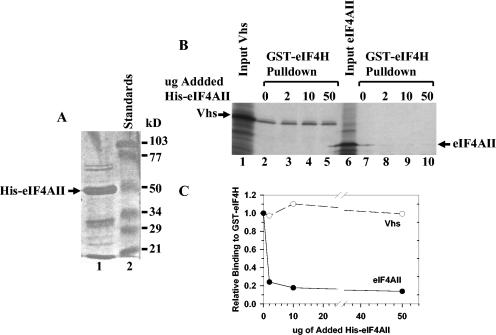
FIG. 9.
eIF4AII does not compete with Vhs for binding to GST-eIF4H. (A) His-tagged eIF4AII was expressed in E. coli and isolated by metal chelate affinity chromatography. An aliquot was analyzed by SDS-PAGE and detected by staining the gel with Coomassie (lane 1). His-eIF4AII is indicated by the arrow. Lane 2 contains molecular mass standards. (B) GST-eIF4H was expressed in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing [35S]methionine-labeled in vitro-translated Vhs (lanes 2 to 5) or full-length eIF4AII (lanes 7 to 10). The incubations also contained 0 μg (lanes 2 and 7), 2 μg (lanes 3 and 8), 10 μg (lanes 4 and 9), or 50 μg (lanes 5 and 10) of the His-eIF4AII preparation shown in panel A. Material that bound GST-eIF4H and was eluted with 10 mM glutathione was analyzed by SDS-PAGE and autoradiography. Aliquots of the input Vhs and eIF4AII were run in lanes 1 and 6, respectively. The positions of the Vhs and full-length eIF4AII polypeptides are shown by arrows. (C) For each of the incubations, the amount of 35S-labeled Vhs or eIF4AII that bound GST-eIF4H was determined by densitometric scanning of the lanes shown in panel B. For each concentration of added His-eIF4AII, the amount of bound 35S-labeled Vhs or eIF4AII was plotted as a fraction of the amount that bound GST-eIF4H in the absence of added His-eIF4AII.