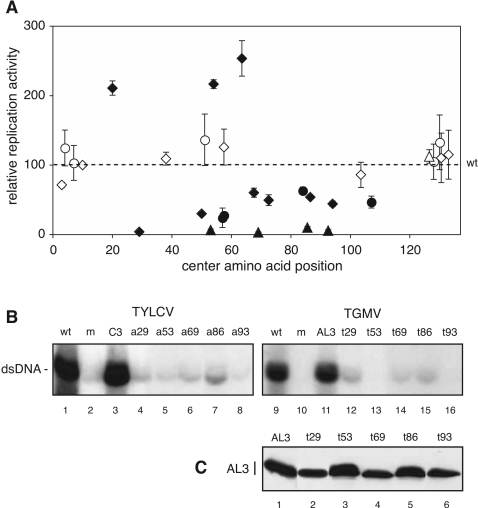
FIG. 3.
Replication enhancement activities of mutated C3 proteins. (A) Tobacco protoplasts were transfected with the TYLCV ΔC3 replicon and wild-type or mutated C3 expression cassettes. Double-stranded viral DNA accumulation was quantified on DNA gel blots by phosphorimage analysis. Relative replication activity was calculated as the ratio of viral DNA in the presence of the mutated expression cassette to that in the presence of the wild-type C3 expression cassettes and plotted against the central position of the mutated motif. Open symbols indicate proteins that displayed wild-type activity. Filled symbols above the dashed line represent proteins that complemented ΔC3 replication significantly better than wild-type C3, while filled symbols below the dashed line represent mutants that showed significantly reduced complementation. Each data point represents at least three independent experiments, with the bars showing 2 standard errors. The statistical significance of each sample relative to the wild type was determined using Student's t test and a cutoff of P < 0.05. The diamonds, triangles, and circles are defined in the Fig. 2 legend. (B) DNA gel blots showing replication complementation for severely impaired C3/AL3 mutants. Wild-type (wt) TYLCV and TGMV A accumulation are shown in lanes 1 and 9, respectively. Lanes 2 to 8 correspond to the ΔC3 replicon, and lanes 10 to 16 contain the ΔAL3 replicon. The expression cassettes in the first panel were an empty 35S cassette (lanes 1 and 2), a wild-type C3 cassette (lane 3), or the mutant C3 cassettes a29 (lane 4), a53 (lane 5), a69 (lane 6), a86 (lane 7), and a93 (lane 8). The expression cassettes in the second panel were an empty 35S cassette (lanes 9 and 10), a wild-type AL3 cassette (lane 11), or the mutant AL3 cassettes t29 (lane 12), t53 (lane 13), t69 (lane 14), t86 (lane 15), and t93 (lane 16). (C) Immunoblot showing TGMV AL3 protein expression in baculovirus-infected SF9 cells. Wild-type AL3 (lane 1), t29 (lane 2), t53 (lane 3), t69 (lane 4), t86 (lane 5), and t93 (lane 6) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected using an anti-AL3 antibody.