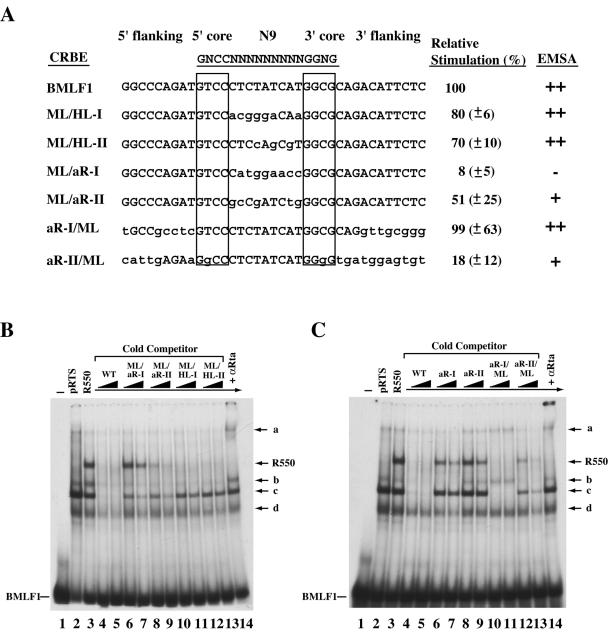
FIG. 3.
Switching the N9 internal sequences among different CRBEs affects their responses to Rta. (A) Summary of DNA binding and transactivation functions of Rta with different mutated CRBEs. The ML/HL-I, ML/HL-II, ML/aR-I, and ML/aR-II constructs retained the flanking and core sequences of the BMLF1 CRBE but had the internal N9 sequences replaced with those from BHLF1-I, BHLF1-II, BaRF1-I, and BaRF1-II, respectively. The aR-I/ML and aR-II/ML mutants retained the internal N9 sequence of the BMLF1 CRBE, but both the core and flanking sequences were replaced with those from BaRF1-I and BaRF1-II CRBEs. The lowercase nucleotides indicate changes in sequence relative to that of the BMLF1 CRBE. Core sequences are boxed. Transcriptional stimulation data were expressed relative to the response of the BMLF1 CRBE, and standard deviations are given in parentheses. Results listed under “EMSA” summarize the capacity to compete for binding of the BMLF1 CRBE. Symbols are as defined in the legend to Fig. 2A. (B and C) EMSAs. Wild-type (WT) or mutant CRBE duplex oligonucleotides were used as competitors in DNA binding reactions. The amount of CRBE duplex oligonucleotides added was 50 ng (lanes 4, 6, 8, 10, and 12) and 100 ng (lanes 5, 7, 9, 11, and 13). +αRta, addition of Rta antibody.