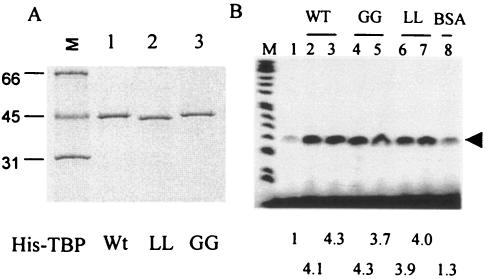
FIG. 8.
Purification and transcriptional activity of GG rTBP and LL rTBP. (A) Recombinant His-tagged wt TBP, GG rTBP, and LL rTBP were expressed in bacteria and purified by Ni++ affinity chromatography. Silver-stained gel of the purified proteins is shown. (B) The transcriptional activity at 3 and 5 μM of each of the wt (lanes 2 and 3), GG (lanes 4 and 5), and LL (lanes 6 and 7) rTBP was measured using a template containing the Ad MLP (lanes 2 to 7) in PV-infected cell extract as previously described (37). Bovine serum albumin (5 μM) was used as a negative control (lane 8). Lane 1 shows transcriptional activity of the infected extract in the absence of any added protein. The numbers at the bottom indicate the increase in stimulation (n-fold) over control as determined by densitometric quantification of the transcript.