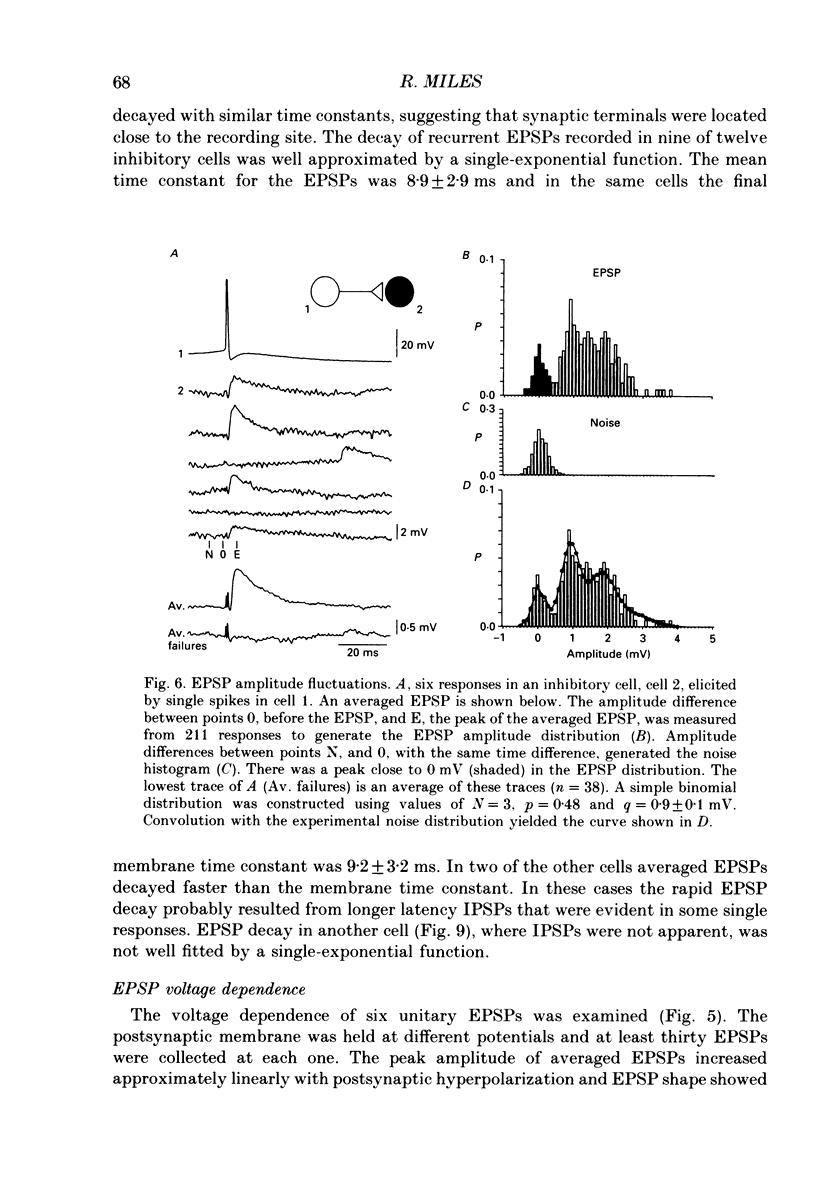
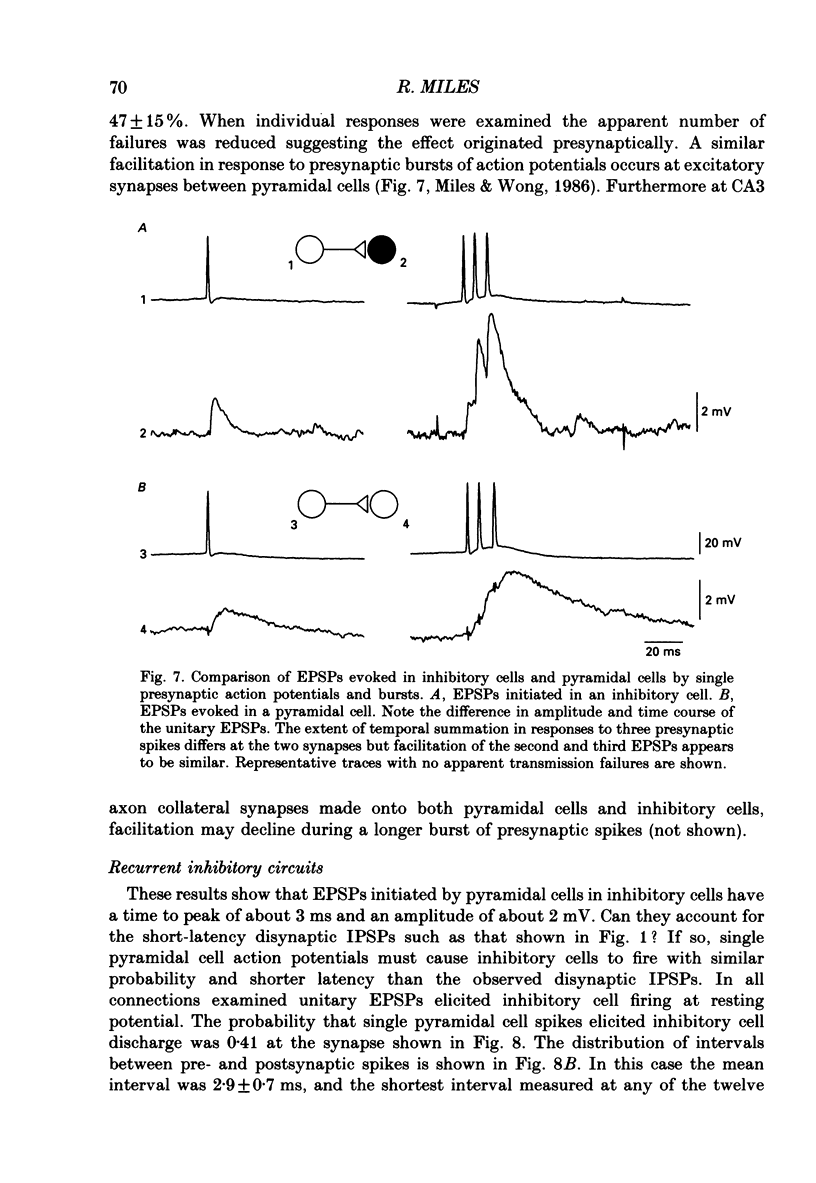
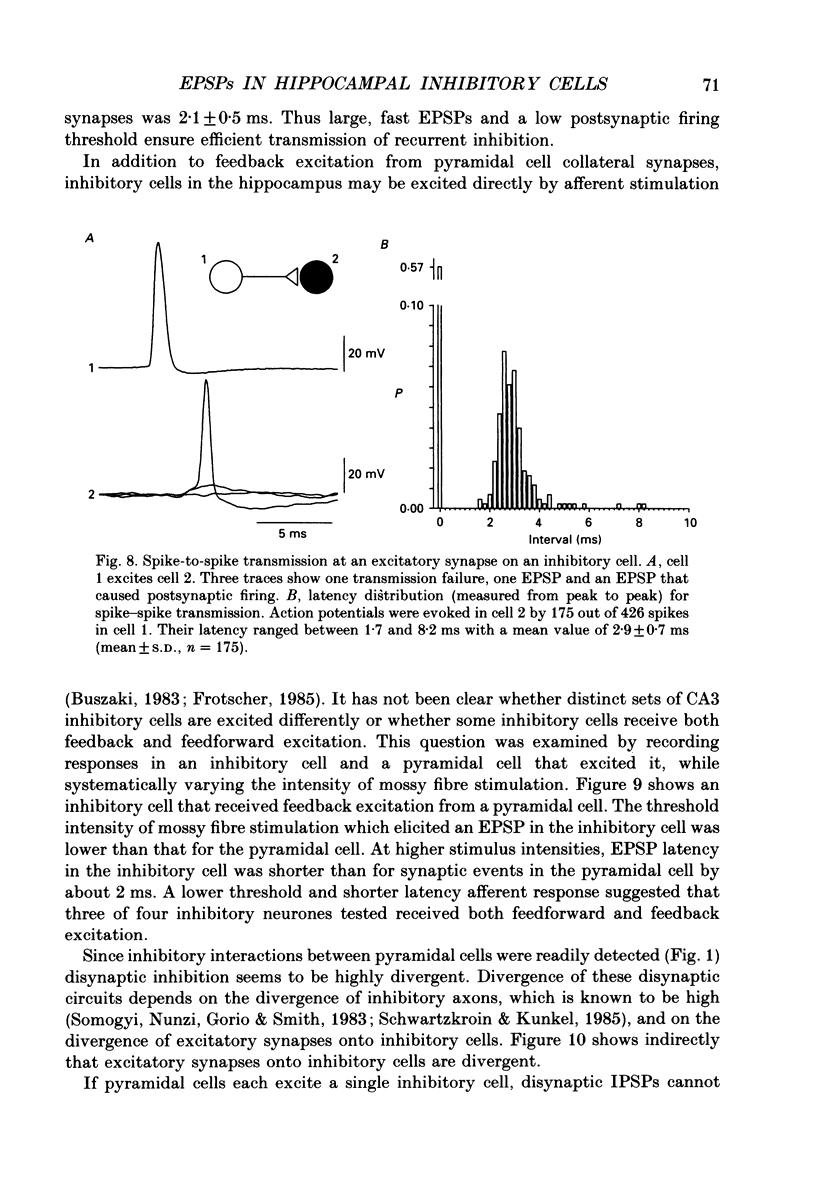
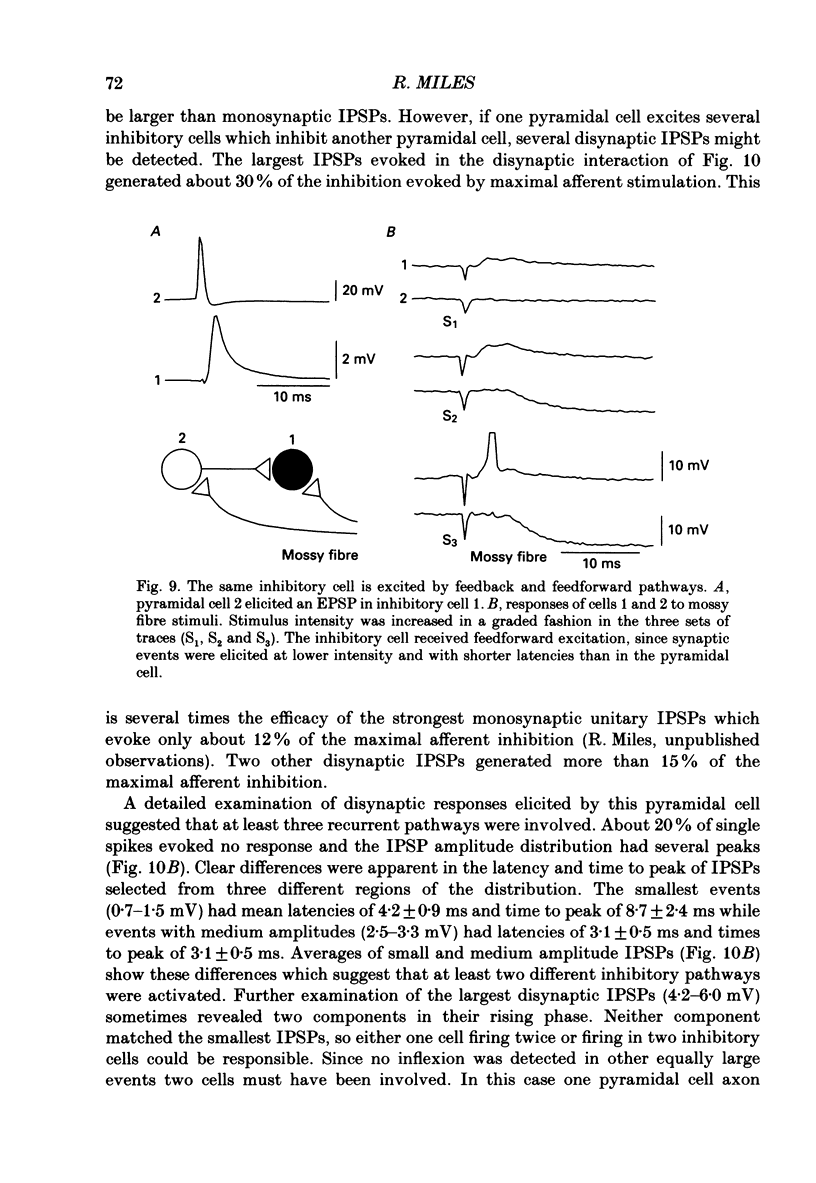
Abstract
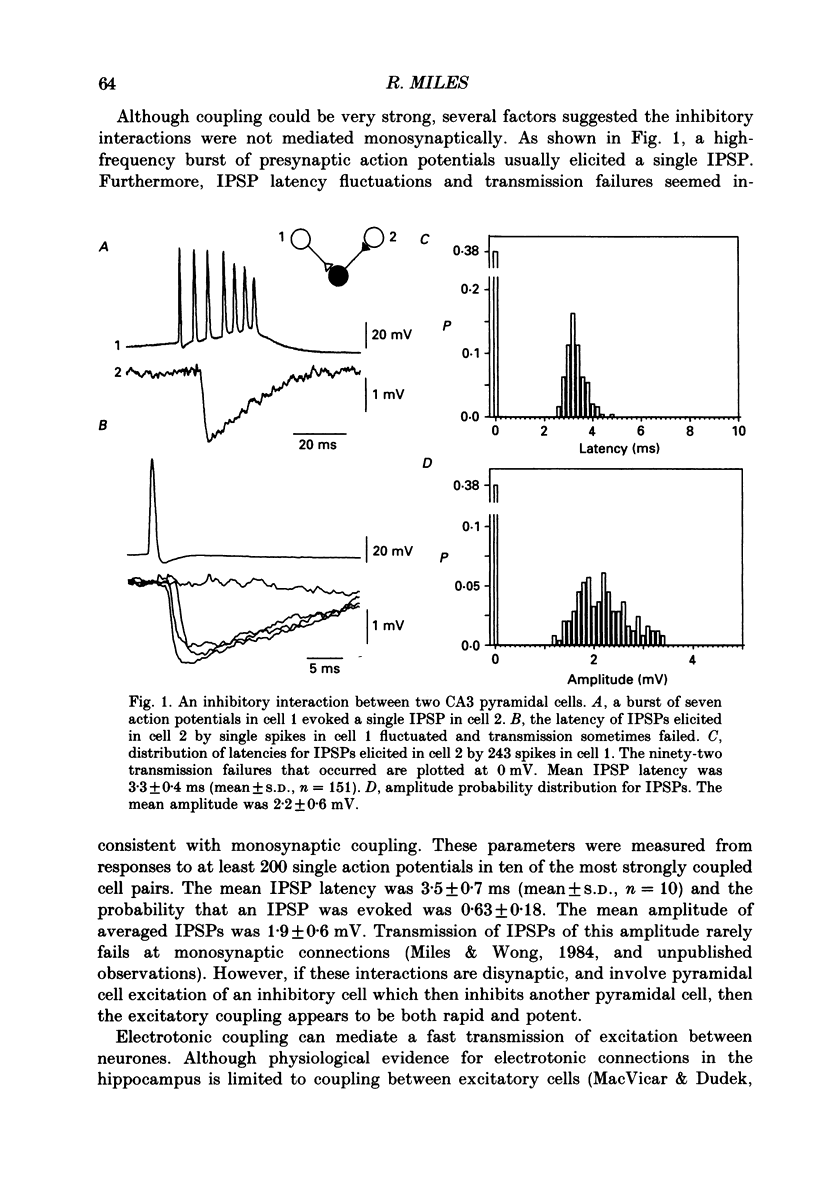
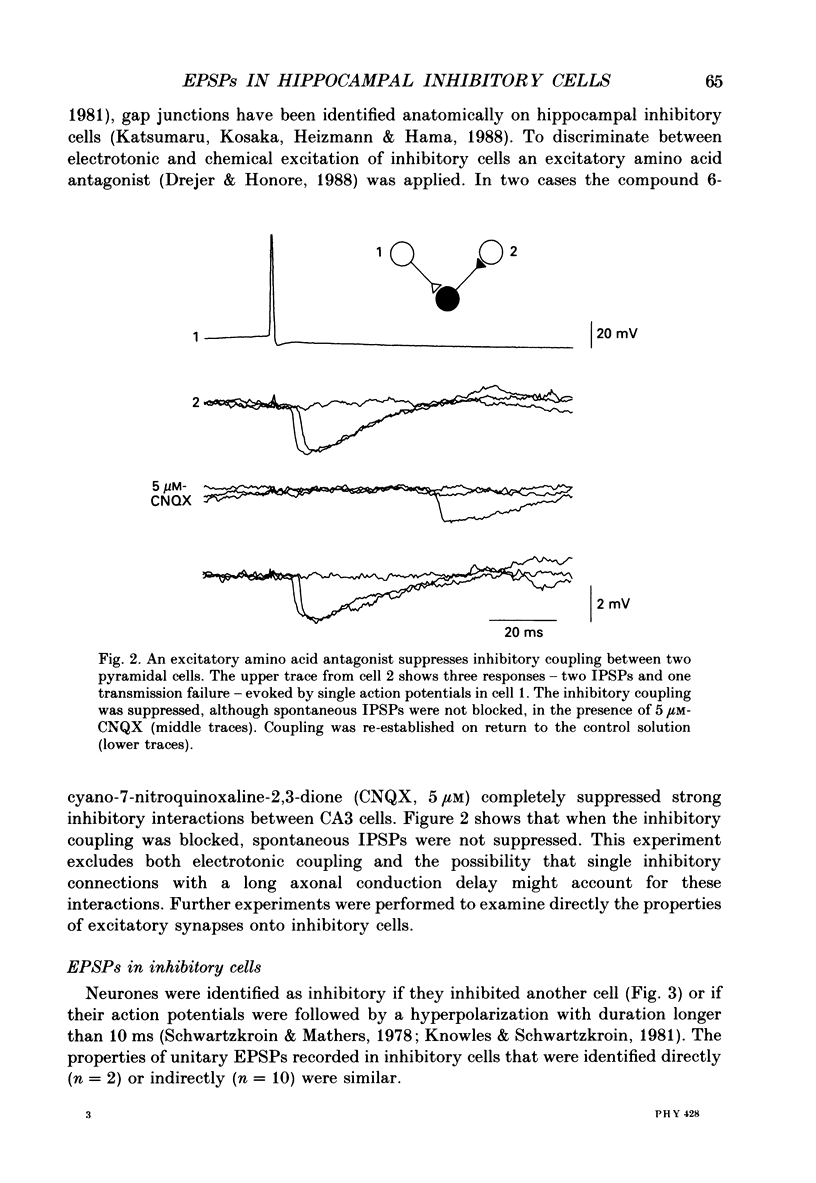
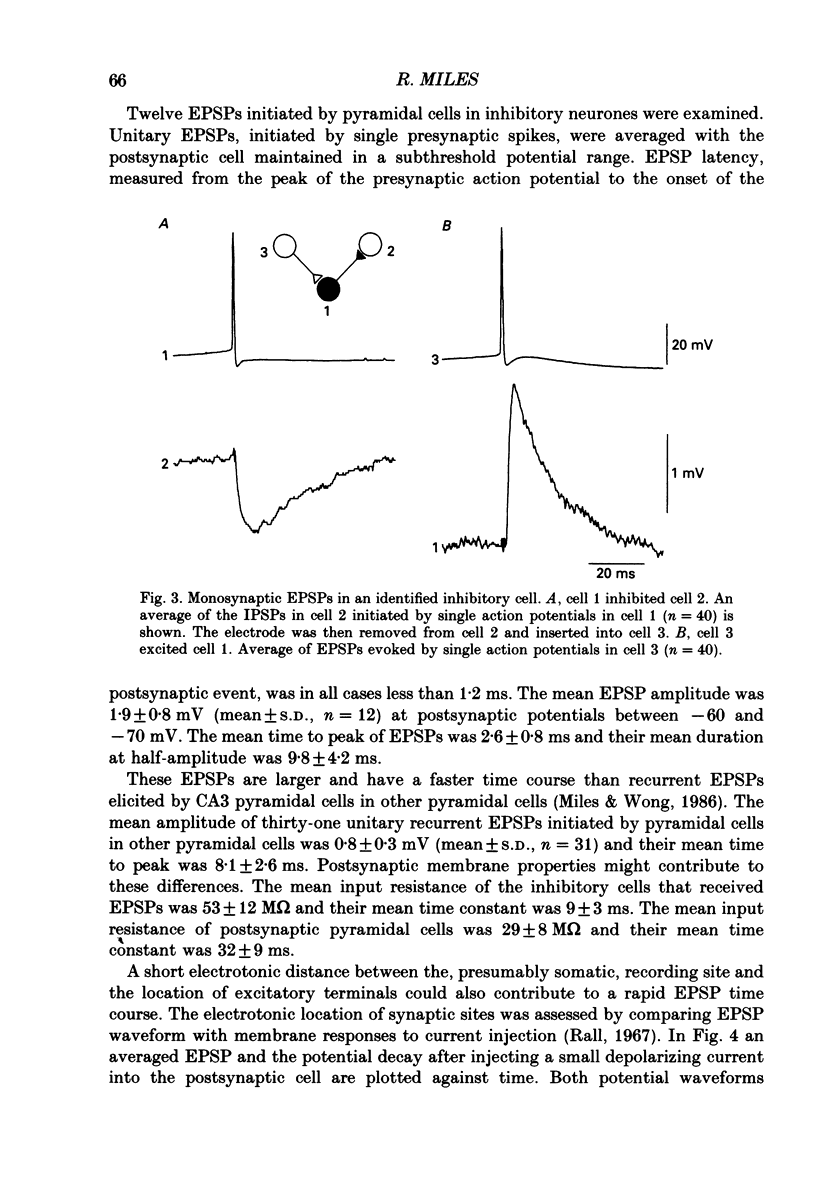
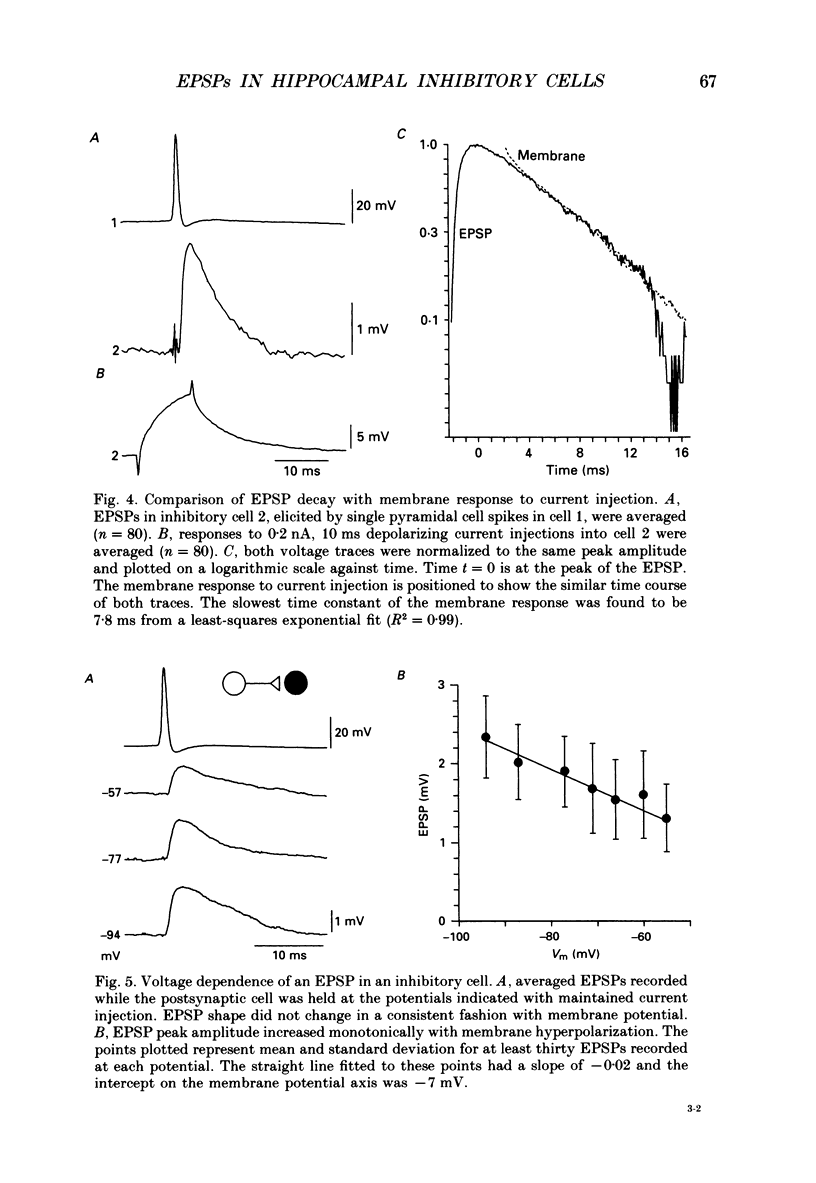
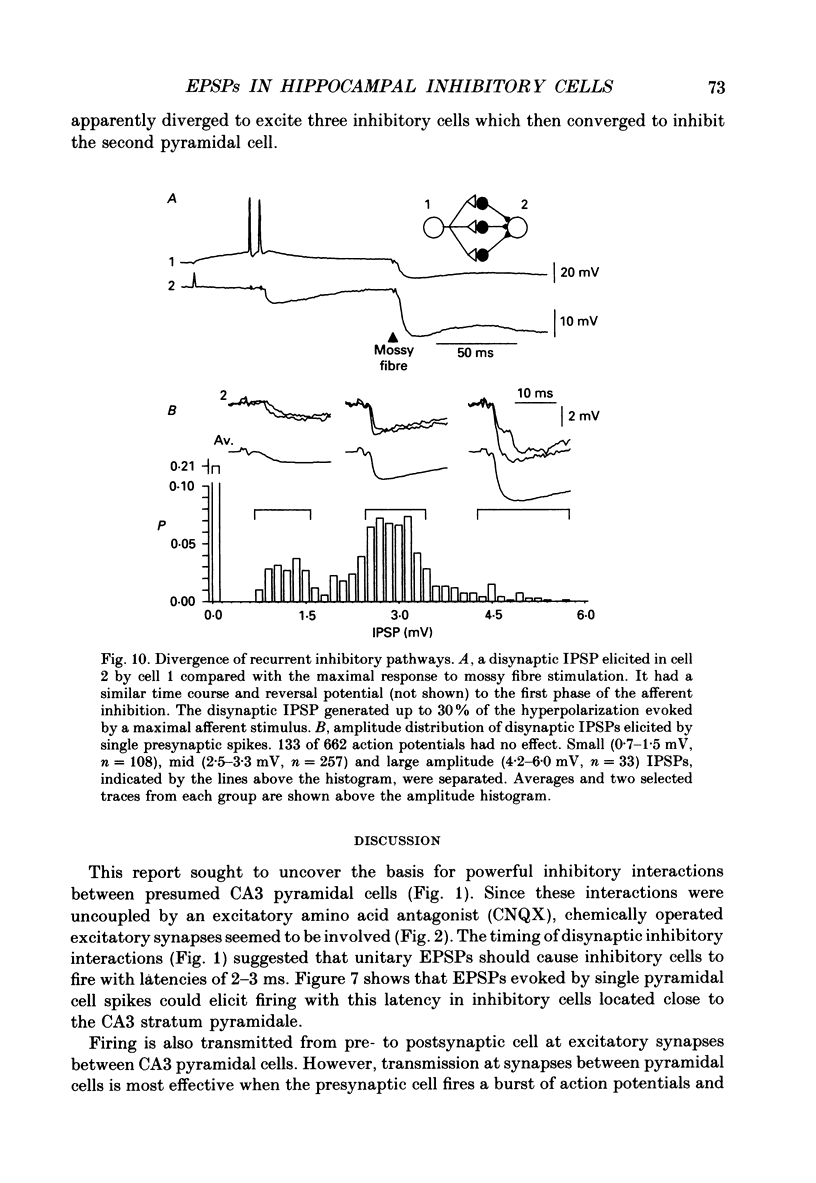
1. In simultaneous recordings from pairs of neurones in hippocampal slices from guinea-pigs, single action potentials fired by CA3 pyramidal cells could initiate inhibitory postsynaptic potentials (IPSPs) in nearby pyramidal cells. 2. The latencies of these IPSPs could be as short as 3 ms. However, they were mediated disynaptically via chemical, excitatory synapses, since inhibitory coupling was suppressed by an excitatory amino acid antagonist. 3. The properties of excitatory synapses made onto inhibitory cells were examined to assess the basis for this strong coupling. Inhibitory cells were identified either by showing that they inhibited another cell or by their characteristic firing pattern. 4. Excitatory postsynaptic potentials (EPSPs) elicited by single pyramidal cell action potentials had a mean amplitude of 1-4 mV and a time to peak of 1.5-4 ms. In most cases they decayed with a time constant similar to that of the inhibitory cell membrane. 5. EPSP amplitude increased with hyperpolarization of the postsynaptic membrane. Membrane polarization had little effect on EPSP shape. 6. EPSPs fluctuated in amplitude and transmission sometimes failed, suggesting transmission was quantal and that few quanta were released. 7. When presynaptic cells were made to fire bursts of action potentials, EPSPs in inhibitory cells were initially potentiated. 8. EPSPs could cause inhibitory cells to fire. The interval between pre- and postsynaptic spikes could be as short as 2.5 ms and the probability of spike transmission could be as high as 0.6. Some inhibitory cells which received feedback excitation were also excited in feedforward fashion by mossy fibre stimuli. 9. One pyramidal cell could activate several disynaptic inhibitory pathways terminating on another pyramidal cell. This suggests that excitatory synapses made by pyramidal cell axon collaterals onto inhibitory cells are divergent. 10. This strong, divergent excitation of inhibitory cells ensures recurrent inhibition is sufficiently widespread, rapid and potent to control the spread of activity by recurrent excitatory connections between CA3 pyramidal cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22(2):131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer J., Honoré T. New quinoxalinediones show potent antagonism of quisqualate responses in cultured mouse cortical neurons. Neurosci Lett. 1988 Apr 22;87(1-2):104–108. doi: 10.1016/0304-3940(88)90153-x. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M. Mossy fibres form synapses with identified pyramidal basket cells in the CA3 region of the guinea-pig hippocampus: a combined Golgi-electron microscope study. J Neurocytol. 1985 Apr;14(2):245–259. doi: 10.1007/BF01258450. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- HAMLYN L. H. An electron microscope study of pyramidal neurons in the Ammon's horn of the rabbit. J Anat. 1963 Apr;97:189–201. [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumaru H., Kosaka T., Heizmann C. W., Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region). Exp Brain Res. 1988;72(2):363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Freund T. F., Maglóczky Z., Whitteridge D., Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64(3):541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Whitteridge D., Somogyi P. Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J Comp Neurol. 1985 Nov 8;241(2):111–137. doi: 10.1002/cne.902410202. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Triller A., Mallet A., Faber D. S. Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science. 1981 Aug 21;213(4510):898–901. doi: 10.1126/science.6266015. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981 Aug 14;213(4509):782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. The statistics of transmitter release at chemical synapses. Int Rev Physiol. 1978;17:49–117. [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Redman S. J., Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. J Neurosci. 1989 Mar;9(3):840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Kunkel D. D. Morphology of identified interneurons in the CA1 regions of guinea pig hippocampus. J Comp Neurol. 1985 Feb 8;232(2):205–218. doi: 10.1002/cne.902320206. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Seress L., Ribak C. E. A combined Golgi-electron microscopic study of non-pyramidal neurons in the CA 1 area of the hippocampus. J Neurocytol. 1985 Oct;14(5):717–730. doi: 10.1007/BF01170824. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Nunzi M. G., Gorio A., Smith A. D. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983 Jan 17;259(1):137–142. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Wong R. K., Schulman L. S., Schneiderman J. H. Models of synchronized hippocampal bursts in the presence of inhibition. II. Ongoing spontaneous population events. J Neurophysiol. 1987 Oct;58(4):752–764. doi: 10.1152/jn.1987.58.4.752. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Turner D. A. Waveform and amplitude characteristics of evoked responses to dendritic stimulation of CA1 guinea-pig pyramidal cells. J Physiol. 1988 Jan;395:419–439. doi: 10.1113/jphysiol.1988.sp016927. [DOI] [PMC free article] [PubMed] [Google Scholar]


