Abstract
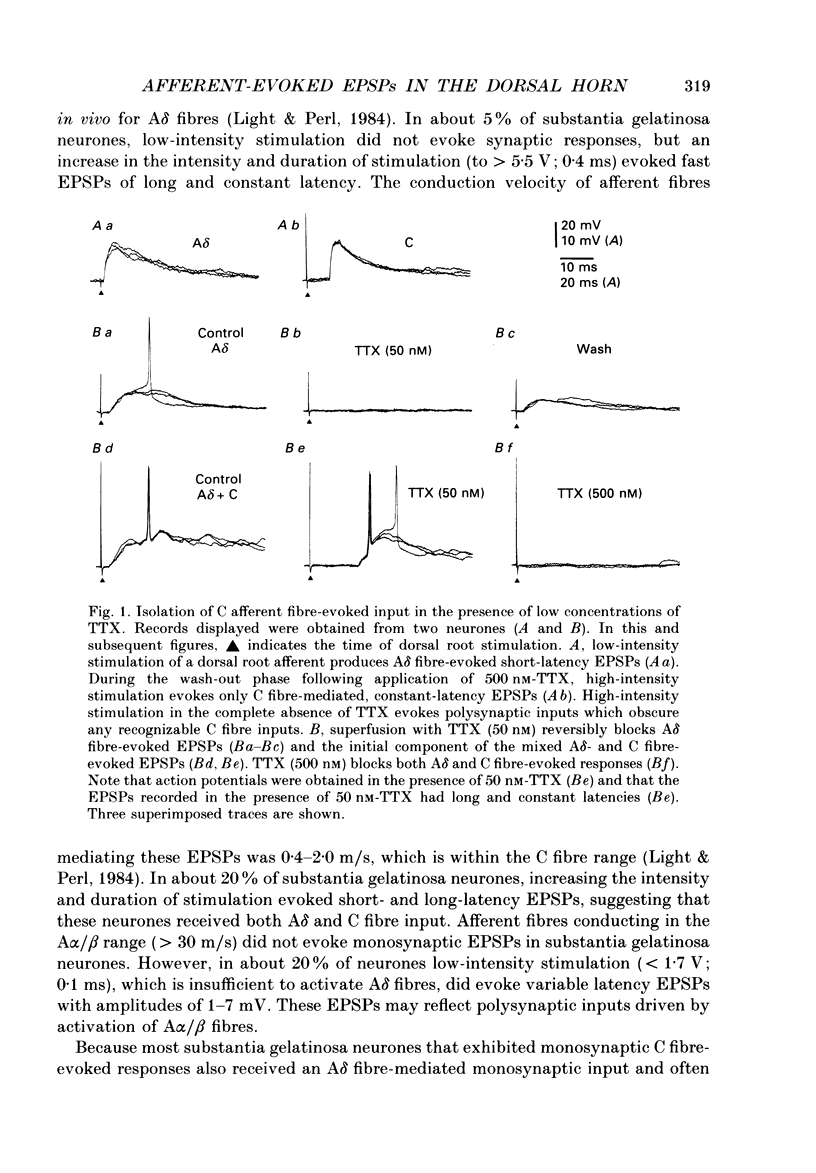
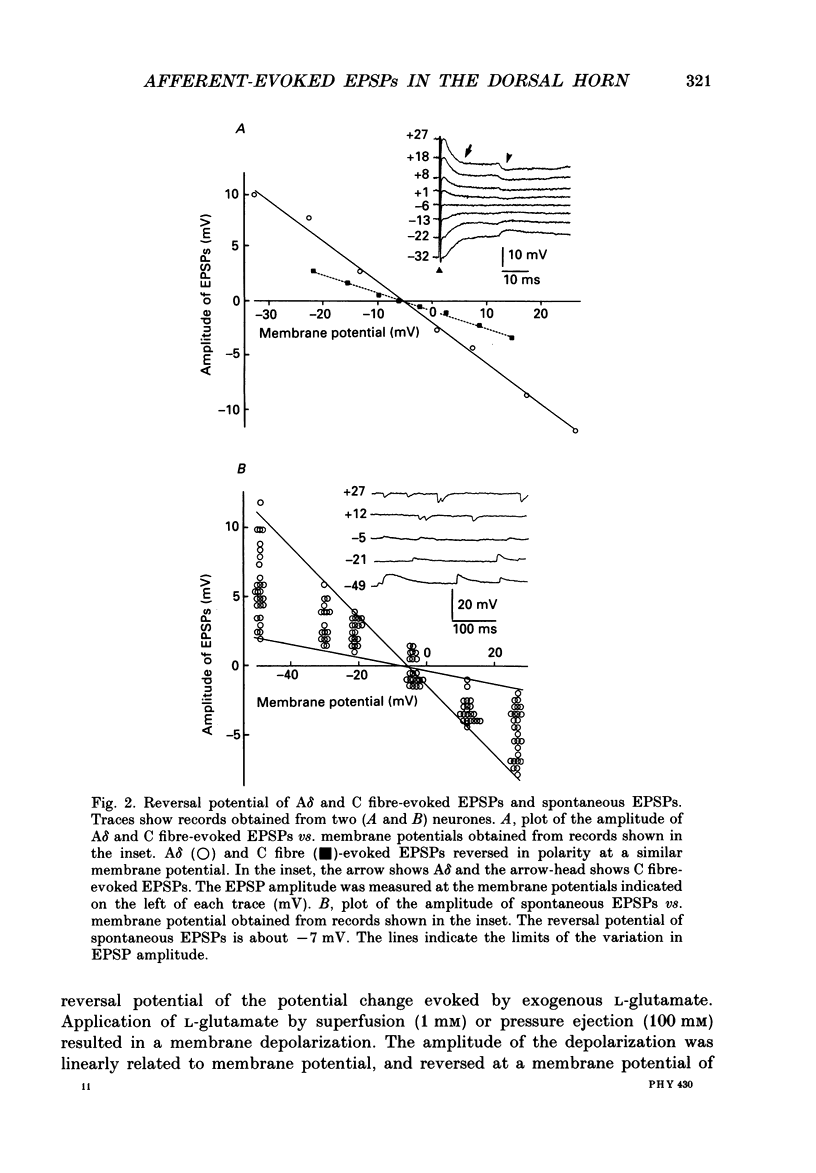
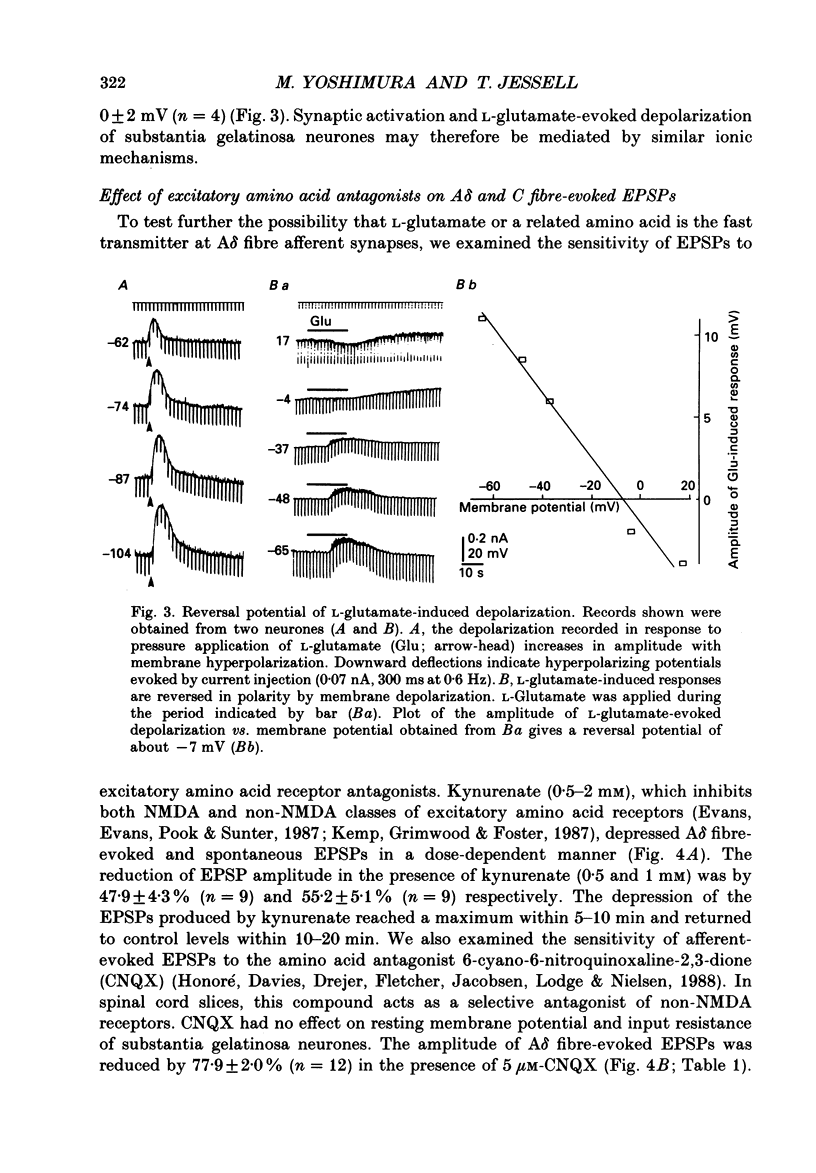
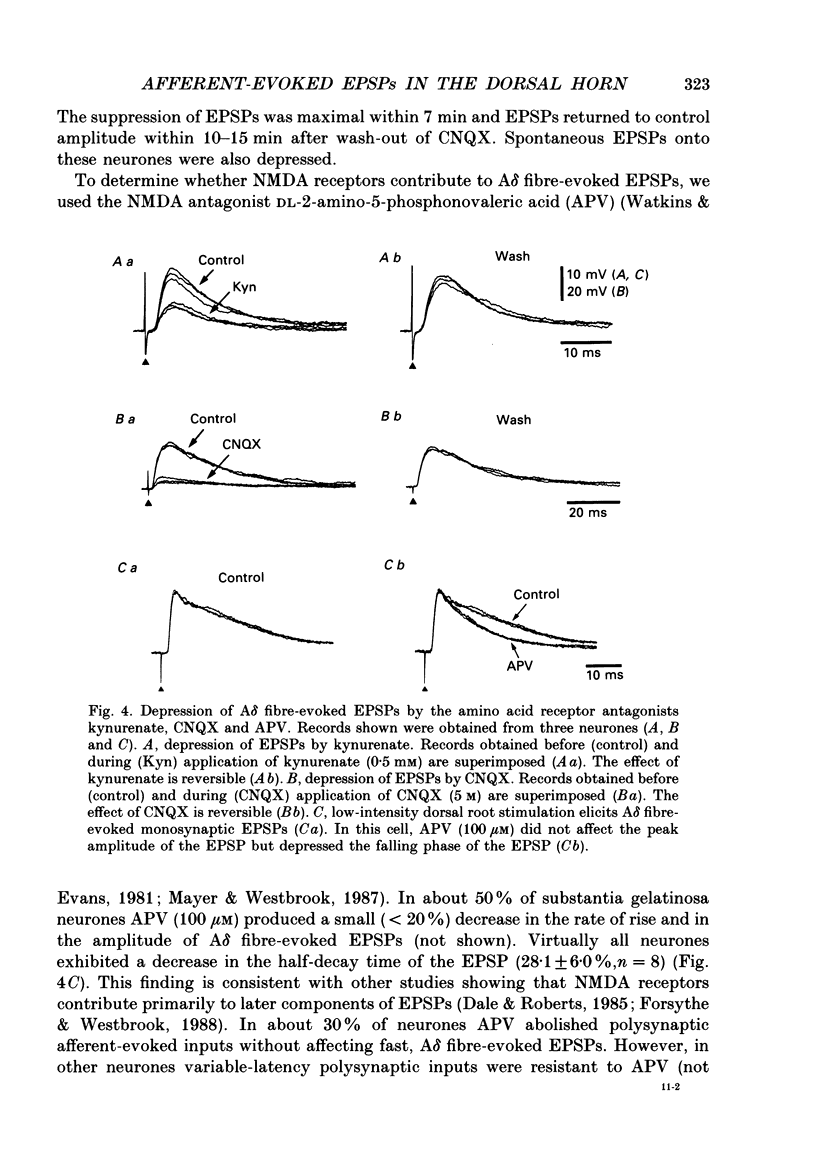
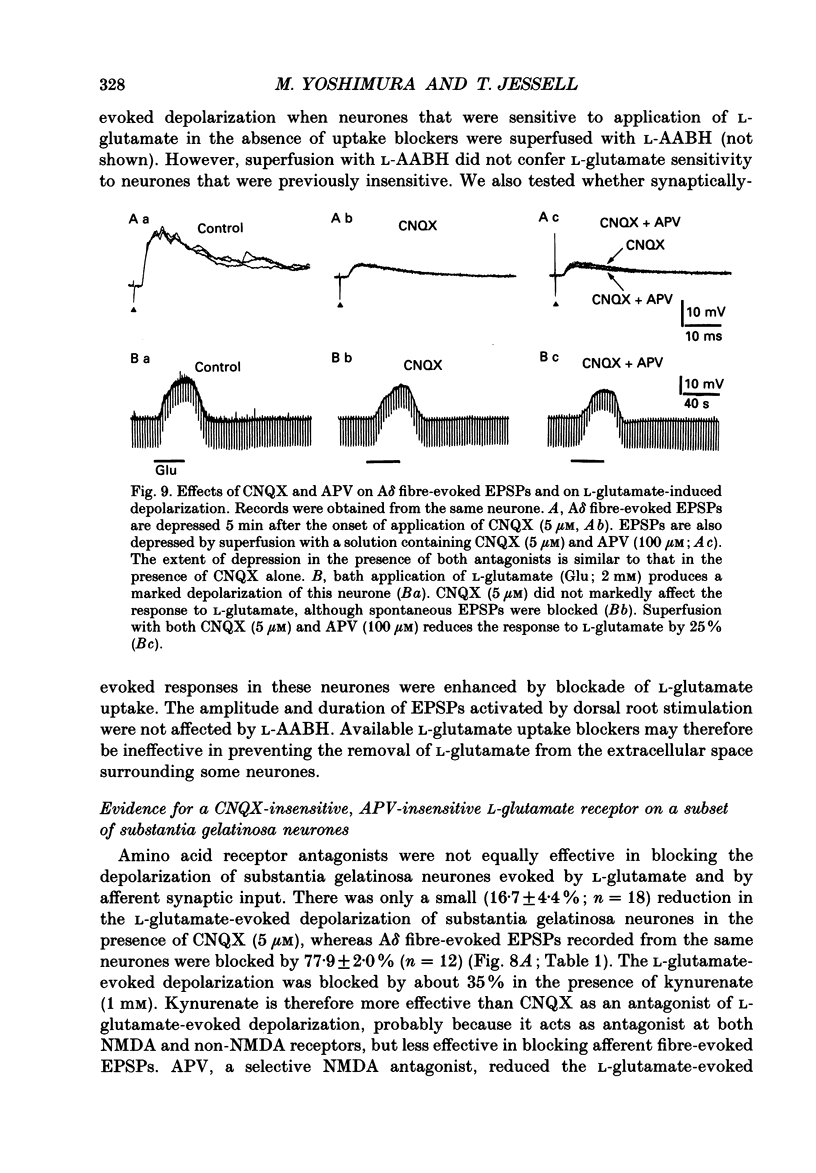
1. Fast excitatory postsynaptic potentials (EPSPs) evoked by stimulation of A delta and C fibres were examined by intracellular recording from substantia gelatinosa (SG) neurones in a transverse slice preparation of adult rat spinal cord. 2. Single low-intensity stimuli applied to the dorsal root activated A delta fibres and evoked monosynaptic EPSPs in 70% of SG neurones. In 5% of SG neurones, increasing the intensity and duration of stimulation evoked solely C fibre-mediated EPSPs. About 20% of neurones received both A delta and C fibre input from primary afferents. 3. Low concentrations of tetrodotoxin (TTX, approximately 50 nM) blocked EPSPs evoked by stimulation of A delta fibres without affecting those evoked by C fibre stimulation. Higher concentrations of TTX (500 nM) also blocked C fibre-evoked responses. 4. EPSPs evoked by A delta and C fibre stimulation reversed in polarity at membrane potentials near 0 mV, similar to the reversal potential of spontaneous EPSPs and of the potential change evoked by exogenous glutamate. 5. A delta and C fibre-evoked EPSPs were depressed by kynurenate and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX); C fibre-evoked EPSPs appeared to be less sensitive. 6. In the presence of TTX, only 50% of SG neurones were depolarized by L-glutamate. However, neurones which exhibited no direct response to L-glutamate received afferent-evoked EPSPs which were sensitive to CNQX. In sensitive neurones, the depolarization evoked by L-glutamate was depressed by only approximately 15% in the presence of CNQX, whereas afferent-evoked EPSPs recorded from the same neurone were almost completely suppressed. Combined application of DL-2-amino-5-phosphonovaleric acid (APV) and CNQX depressed the response to L-glutamate by only approximately 25%. 7. These findings suggest that A delta and C fibres use L-glutamate or a related amino acid as a transmitter at synapses with substantia gelatinosa neurones. The postsynaptic actions of this transmitter are mediated predominantly by non N-methyl-D-aspartic acid (NMDA) receptors. The failure of CNQX and APV to completely block the L-glutamate-evoked depolarization of substantia gelatinosa neurones raises the possibility that exogenously applied L-glutamate activates a non-NMDA receptor distinct from that which mediates the actions of the synaptically released afferent transmitter.
Full text
PDF











Selected References
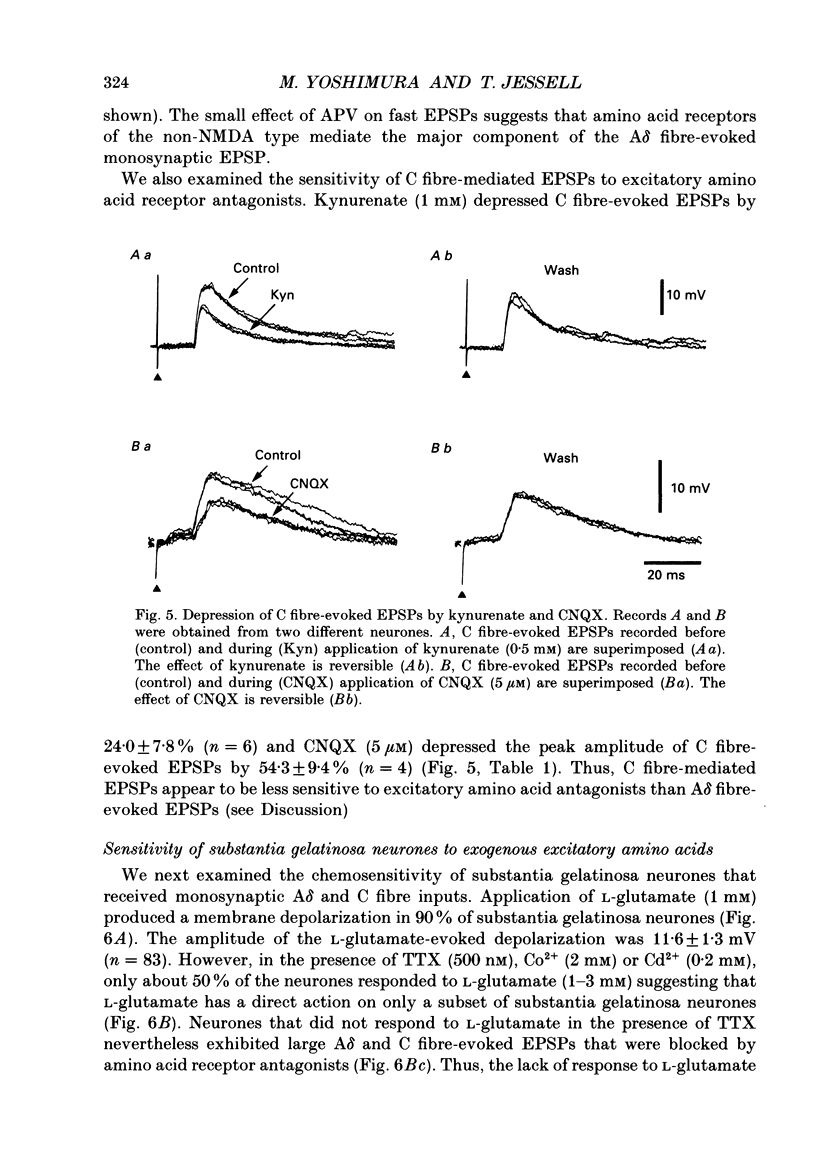
These references are in PubMed. This may not be the complete list of references from this article.
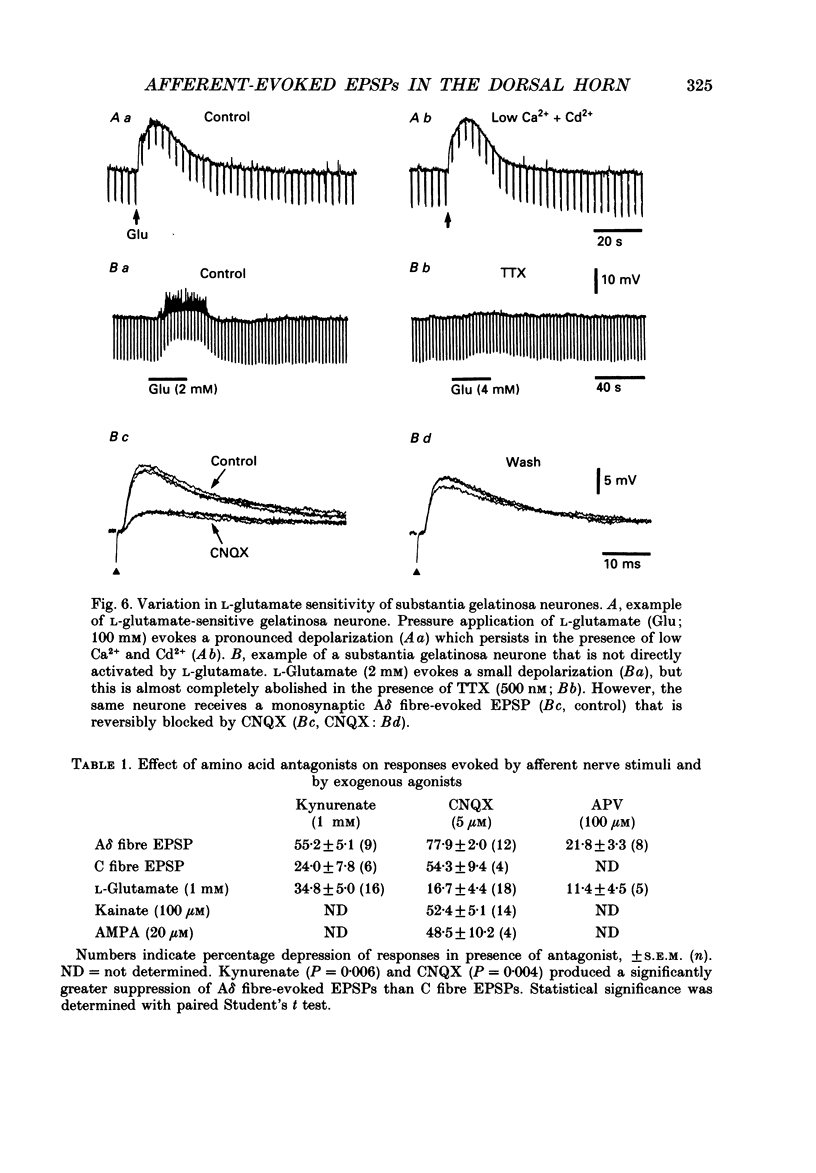
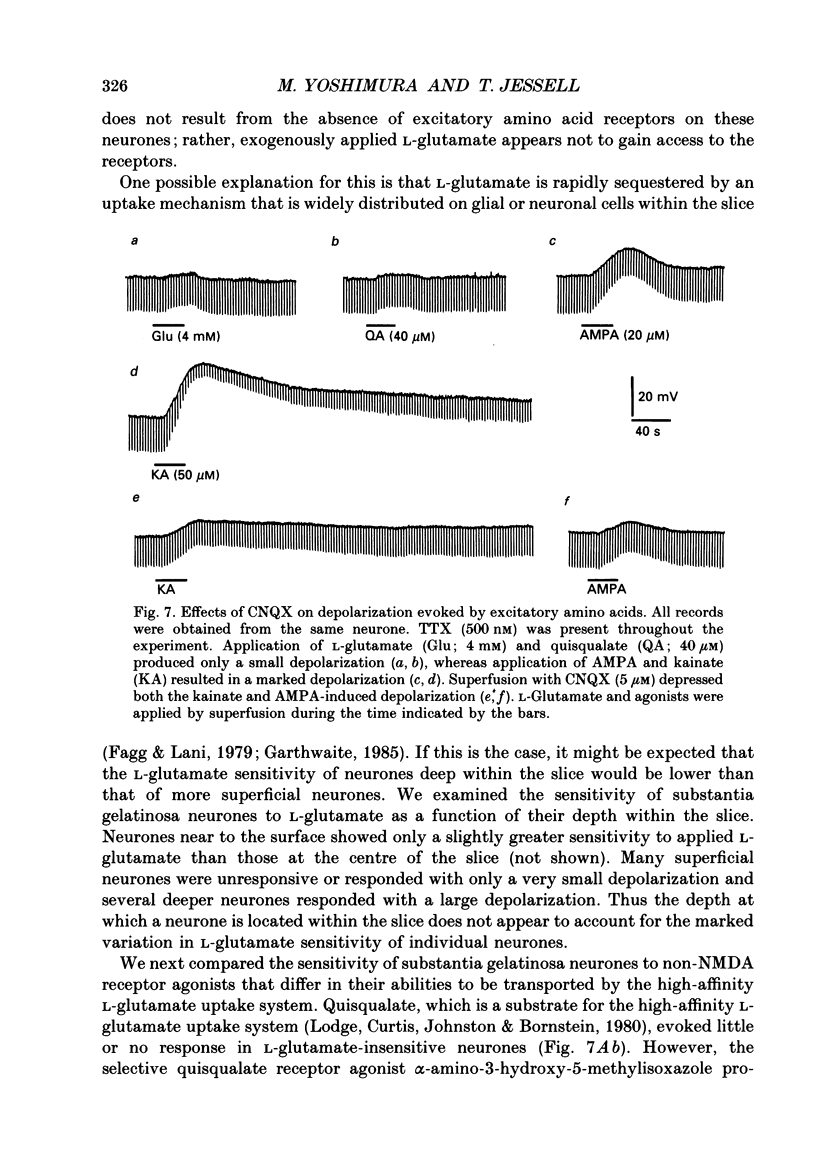
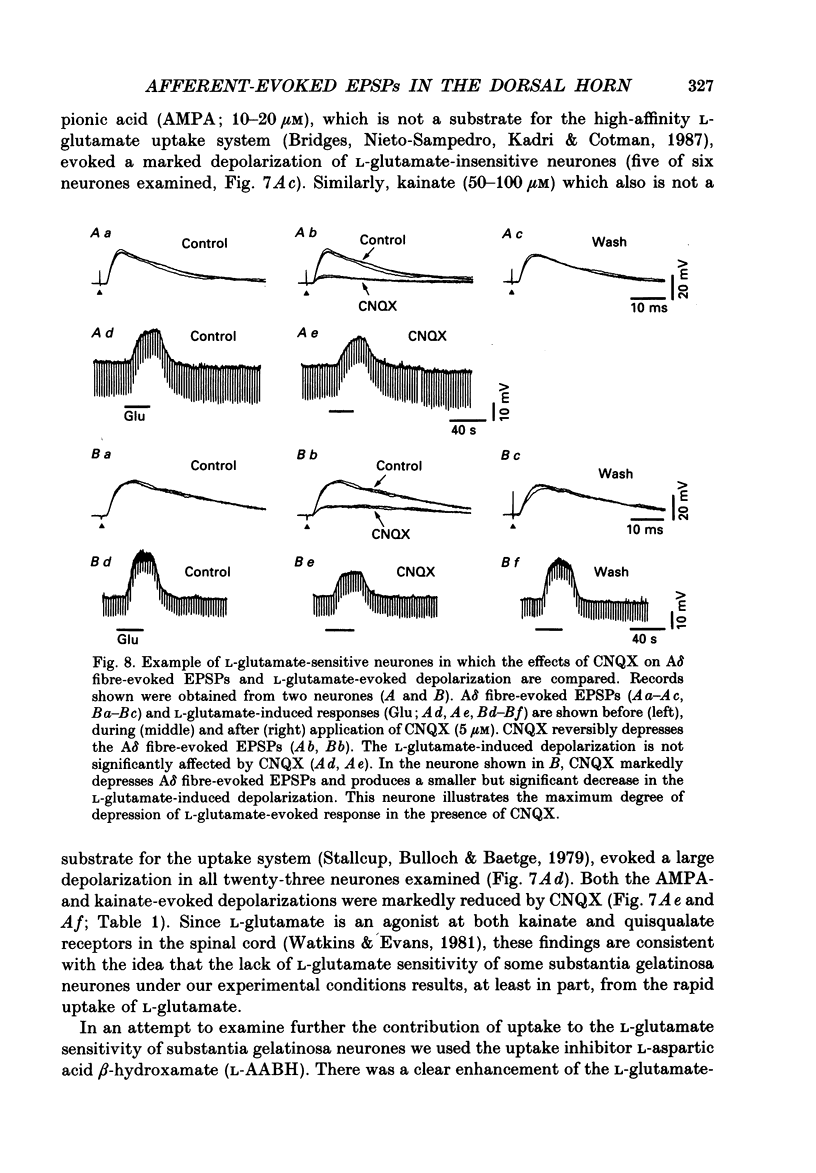
- Bridges R. J., Nieto-Sampedro M., Kadri M., Cotman C. W. A novel chloride-dependent L-[3H]glutamate binding site in astrocyte membranes. J Neurochem. 1987 Jun;48(6):1709–1715. doi: 10.1111/j.1471-4159.1987.tb05727.x. [DOI] [PubMed] [Google Scholar]
- Brown A. G. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982 Apr;67(2):193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Ascending projections of nociceptor-driven Lamina I neurones in the cat. Exp Brain Res. 1979 Mar 9;35(1):135–149. doi: 10.1007/BF00236790. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Evans S. J., Pook P. C., Sunter D. C. A comparison of excitatory amino acid antagonists acting at primary afferent C fibres and motoneurones of the isolated spinal cord of the rat. Br J Pharmacol. 1987 Jul;91(3):531–537. doi: 10.1111/j.1476-5381.1987.tb11246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Lane J. D. The uptake and release of putative amino acid neurotransmitters. Neuroscience. 1979;4(8):1015–1036. doi: 10.1016/0306-4522(79)90185-4. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe R. E., Perl E. R. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci U S A. 1984 Nov;81(21):6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. With an appendix: diffusion of transported amino acids into brain slices. Br J Pharmacol. 1985 May;85(1):297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G., Randić M. Participation of excitatory amino acid receptors in the slow excitatory synaptic transmission in the rat spinal dorsal horn in vitro. Neurosci Lett. 1989 Nov 20;106(1-2):220–228. doi: 10.1016/0304-3940(89)90229-2. [DOI] [PubMed] [Google Scholar]
- Giesler G. J., Jr, Menétrey D., Basbaum A. I. Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol. 1979 Mar 1;184(1):107–126. doi: 10.1002/cne.901840107. [DOI] [PubMed] [Google Scholar]
- Gobel S., Falls W. M., Humphrey E. Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: candidates for the terminal axonal arbors of primary neurons with unmyelinated (C) axons. J Neurosci. 1981 Oct;1(10):1163–1179. doi: 10.1523/JNEUROSCI.01-10-01163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum S. L. The spinothalamic system of the rat. I. Locations of cells of origin. J Comp Neurol. 1986 May 8;247(2):159–180. doi: 10.1002/cne.902470204. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. E., Thompson S. W., Urban L., Woolf C. J. The responses recorded in vitro of deep dorsal horn neurons to direct and orthodromic stimulation in the young rat spinal cord. Neuroscience. 1988 Oct;27(1):231–242. doi: 10.1016/0306-4522(88)90233-3. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978 Feb 1;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Light A. R., Perl E. R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979 Jul 15;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lima D., Coimbra A. The spinothalamic system of the rat: structural types of retrogradely labelled neurons in the marginal zone (lamina I). Neuroscience. 1988 Oct;27(1):215–230. doi: 10.1016/0306-4522(88)90232-1. [DOI] [PubMed] [Google Scholar]
- Lodge D., Curtis D. R., Johnston G. A., Bornstein J. C. In vivo inactivation of quisqualate: studies in the cat spinal cord. Brain Res. 1980 Jan 27;182(2):491–495. doi: 10.1016/0006-8993(80)91211-1. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Pun R. Y., Neale E. A., Nelson P. G. Synaptic interactions between mammalian central neurons in cell culture. I. Reversal potential for excitatory postsynaptic potentials. J Neurophysiol. 1983 Jun;49(6):1428–1441. doi: 10.1152/jn.1983.49.6.1428. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Murase K., Nedeljkov V., Randić M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 1982 Feb 18;234(1):170–176. doi: 10.1016/0006-8993(82)90483-8. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol. 1984 Jan;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P. The termination of primary afferents within the rat dorsal horn: evidence for rearrangement following capsaicin treatment. J Comp Neurol. 1983 Aug 1;218(2):145–158. doi: 10.1002/cne.902180203. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Coimbra A. Two types of synaptic glomeruli and their distribution in laminae I-III of the rat spinal cord. J Comp Neurol. 1982 Aug 1;209(2):176–186. doi: 10.1002/cne.902090205. [DOI] [PubMed] [Google Scholar]
- Roberts P. J. The release of amino acids with proposed neurotransmitter function from the cuneate and gracile nuclei of the rat in vivo. Brain Res. 1974 Mar 8;67(3):419–428. doi: 10.1016/0006-8993(74)90491-0. [DOI] [PubMed] [Google Scholar]
- Réthelyi M. Preterminal and terminal axon arborizations in the substantia gelatinosa of cat's spinal cord. J Comp Neurol. 1977 Apr 1;172(3):511–521. doi: 10.1002/cne.901720307. [DOI] [PubMed] [Google Scholar]
- Schneider S. P., Perl E. R. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neurosci. 1988 Jun;8(6):2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Bulloch K., Baetge E. E. Coupled transport of glutamate and sodium in a cerebellar nerve cell line. J Neurochem. 1979 Jan;32(1):57–65. doi: 10.1111/j.1471-4159.1979.tb04509.x. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Lee C. L., Perl E. R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986 Oct 17;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Terui N., Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989 Oct;62(4):834–840. doi: 10.1152/jn.1989.62.4.834. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2834–2838. doi: 10.1073/pnas.85.8.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherer M. M., Seybold V. S. Immunohistochemical studies of substance P, cholecystokinin-octapeptide and somatostatin in dorsal root ganglia of the rat. Neuroscience. 1985 Feb;14(2):593–605. doi: 10.1016/0306-4522(85)90313-6. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]


