Abstract
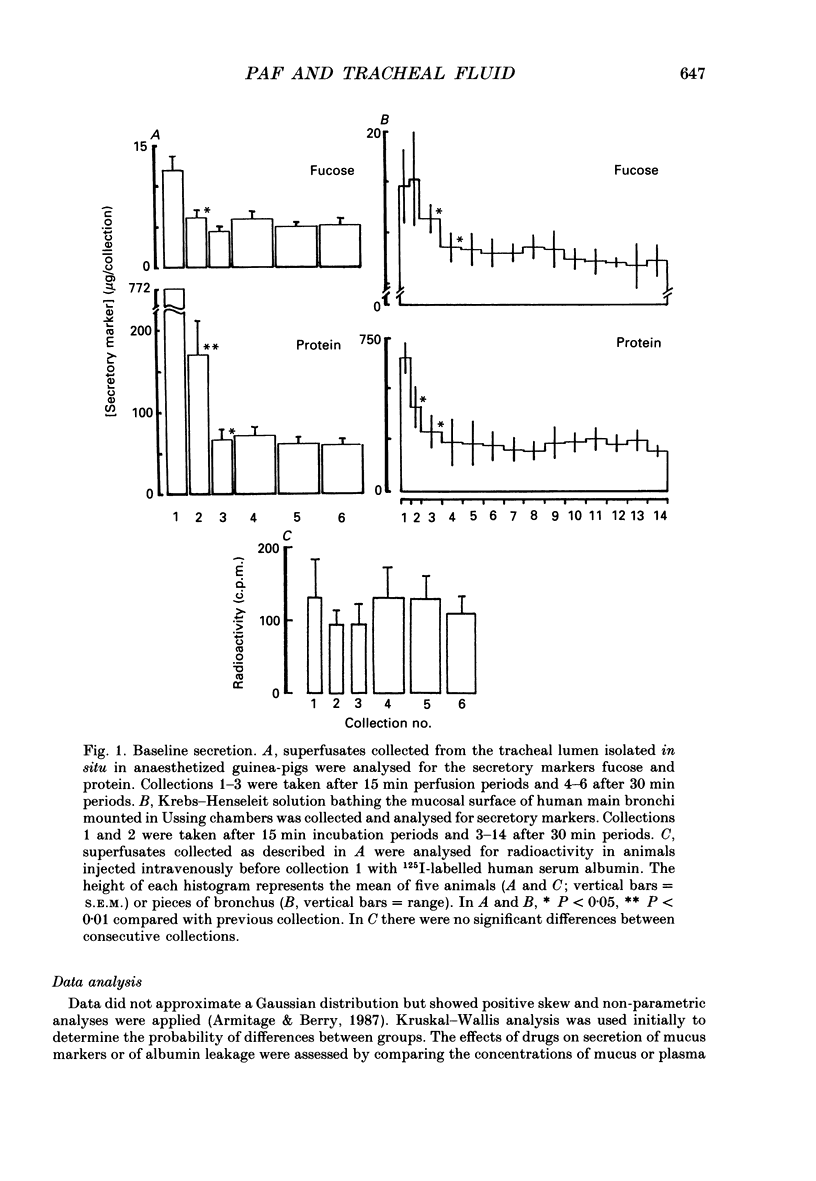
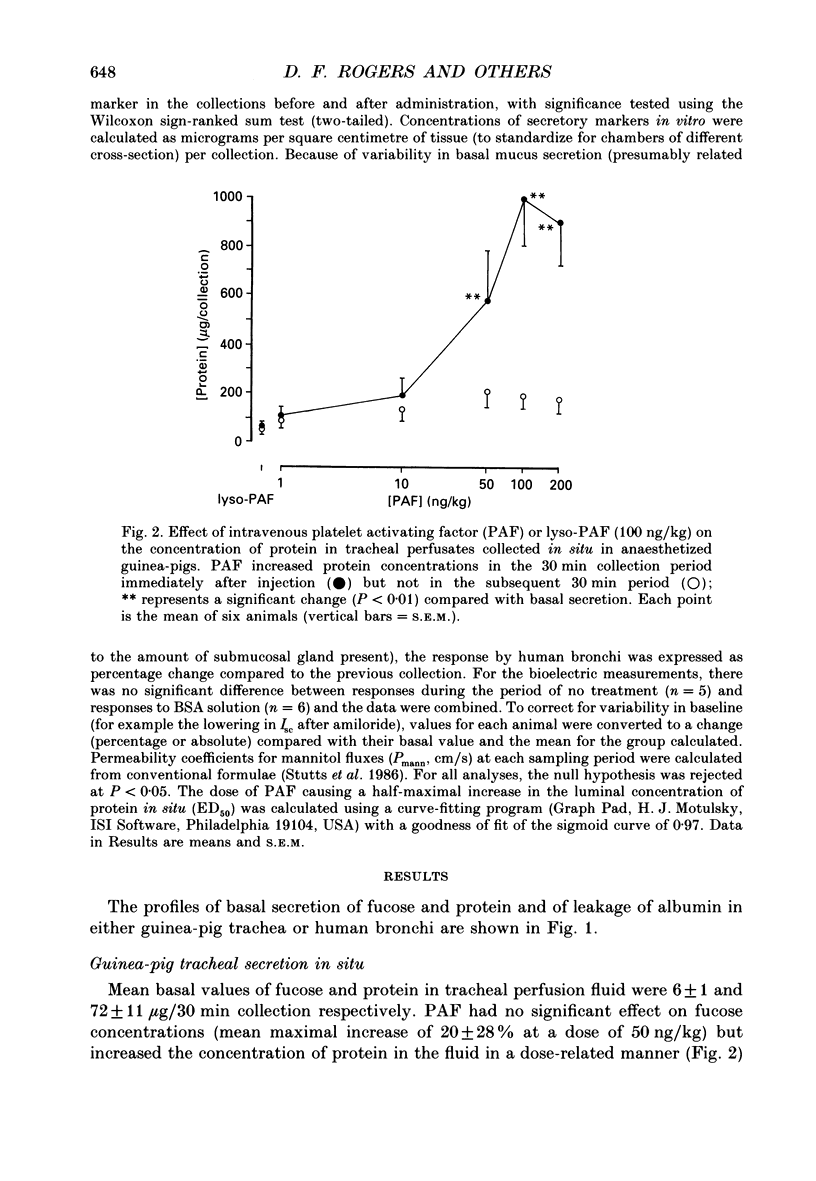
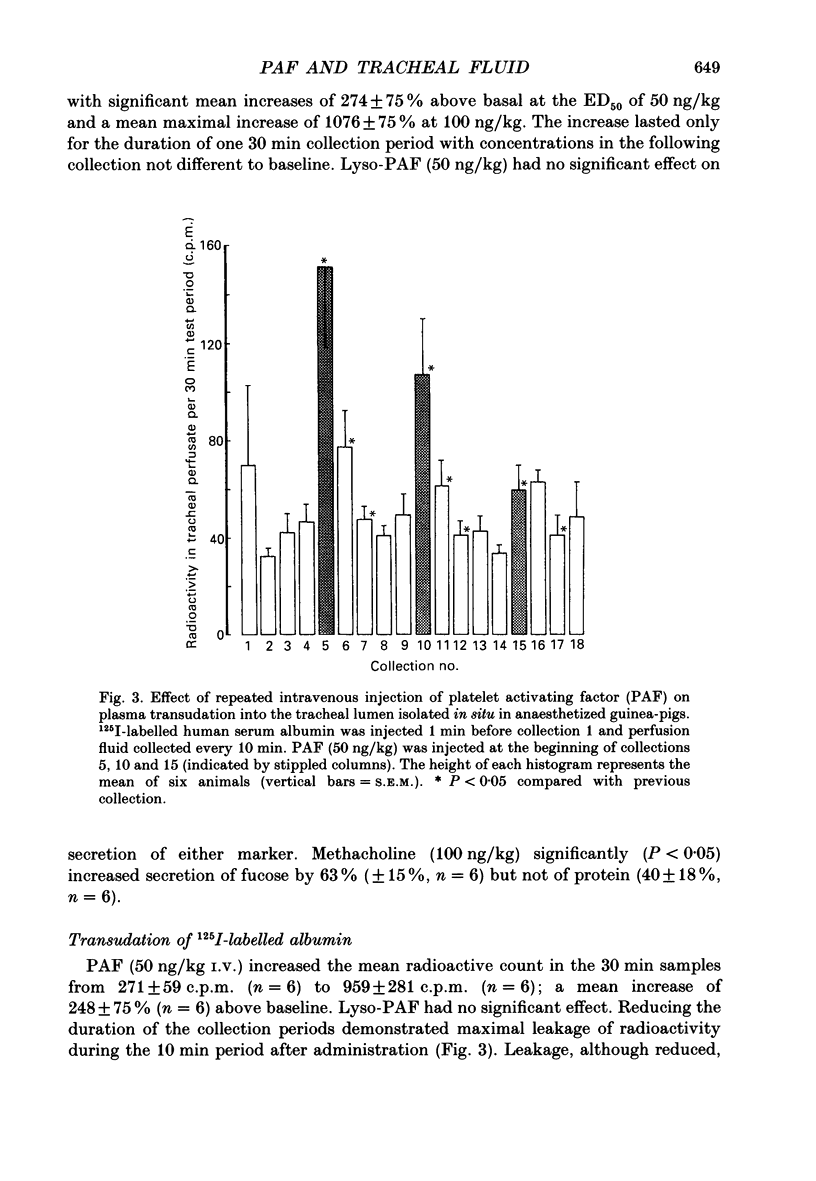
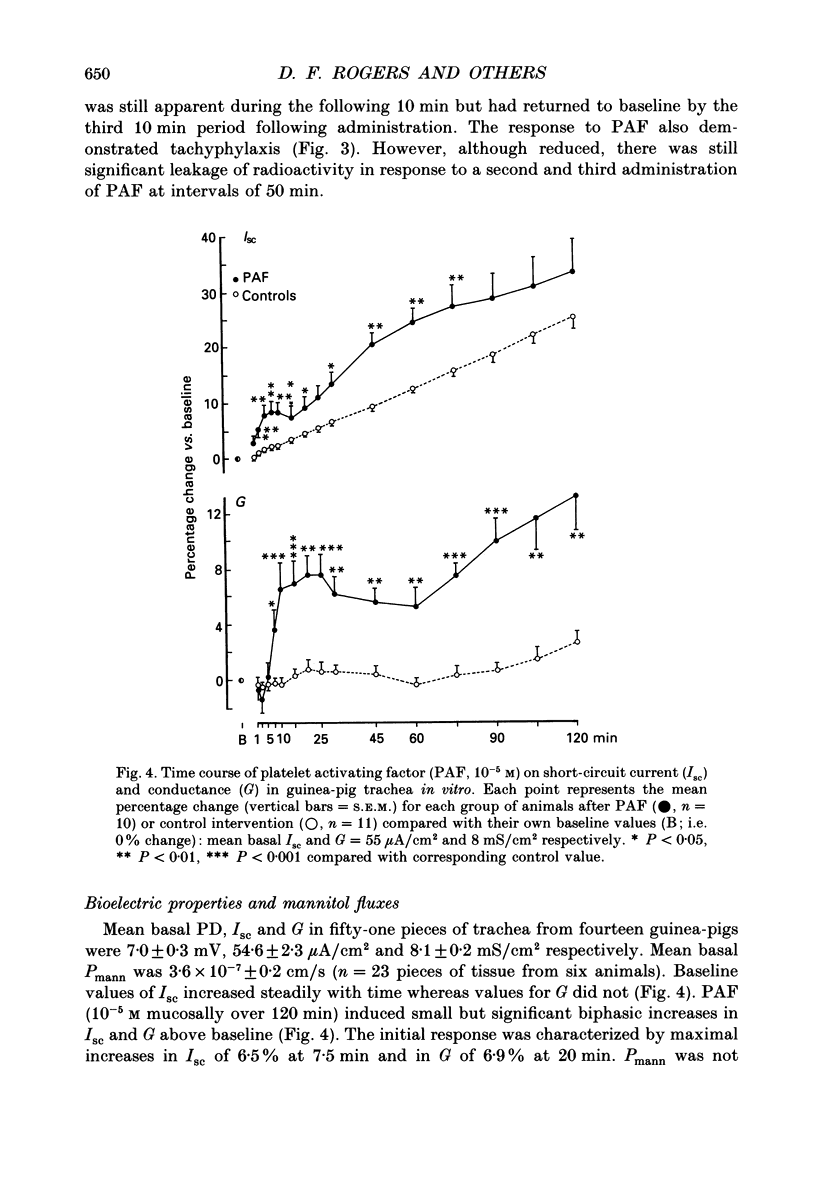
1. We studied the effect of platelet activating factor (PAF) on leakage of albumin, and secretion of fucose (a marker for mucus glycoprotein) and protein into the tracheal lumen of the guinea-pig isolated in situ, and on bioelectric properties and fluxes of mannitol in vitro. We also studied the effect of PAF on mucus secretion in human bronchi in vitro. 2. In guinea-pig, intravenous PAF markedly increased the luminal concentration of protein but did not significantly increase fucose concentrations. Increased albumin leakage (274% above controls at a dose of 50 ng/kg PAF) was associated with the increased luminal content of protein (248% above controls at the same dose of PAF). 3. Leakage of albumin was maximal 10 min after PAF, was significantly reduced by 20 min and had returned to baseline by 30 min. This pattern of leakage could be repeated with successive administrations of PAF. 4. PAF induced small but significant biphasic changes in bioelectric properties in vitro. The initial response was rapid in onset and characterized by maximal increases in short-circuit current (Isc) of 6.5% above controls at 7.5 min and in conductance (G) of 7% at 20 min. Both responses were blocked by the PAF receptor antagonist WEB 2086. Amiloride blocked the increase in Isc. Permeability of the tissue to mannitol (Pmann) was unaltered. The delayed response was characterized by maximal increases in Isc and G of 10% above controls at 60-90 min which were not significantly affected by WEB 2086 or amiloride. Pmann was increased by 38% at 90 min. 5. PAF increased fucose secretion in human bronchi in vitro. 6. Lyso-PAF in vitro caused changes similar to those induced by PAF on bioelectric properties and mucus secretion, but had no significant effects in vivo. 7. Light microscopy showed no evidence of epithelial disruption in animals given intravenous PAF at a dose causing significant albumin transudation. 8. We conclude that PAF increases the protein content of guinea-pig tracheal fluid principally by inducing plasma leakage rather than mucus secretion and that the small changes in ion transport and epithelial conductance may reduce the tendency to epithelial disruption during plasma leakage.
Full text
PDF



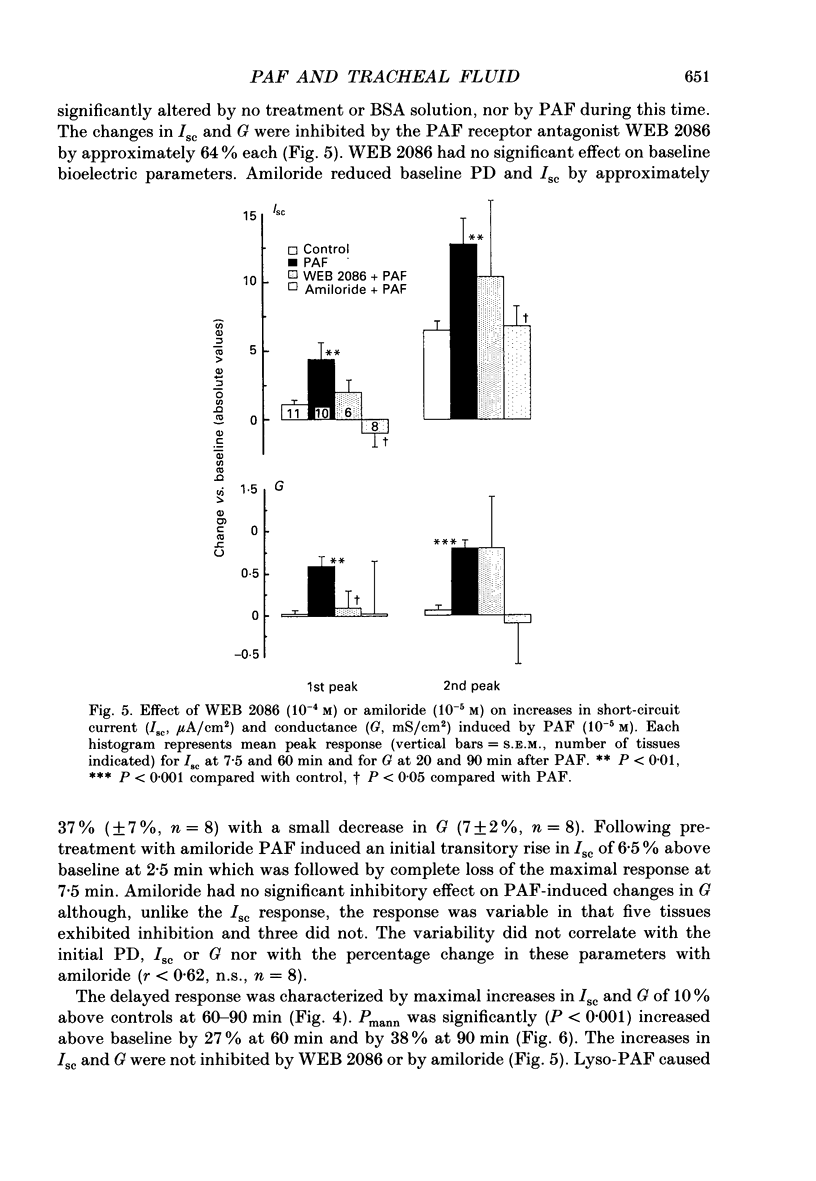
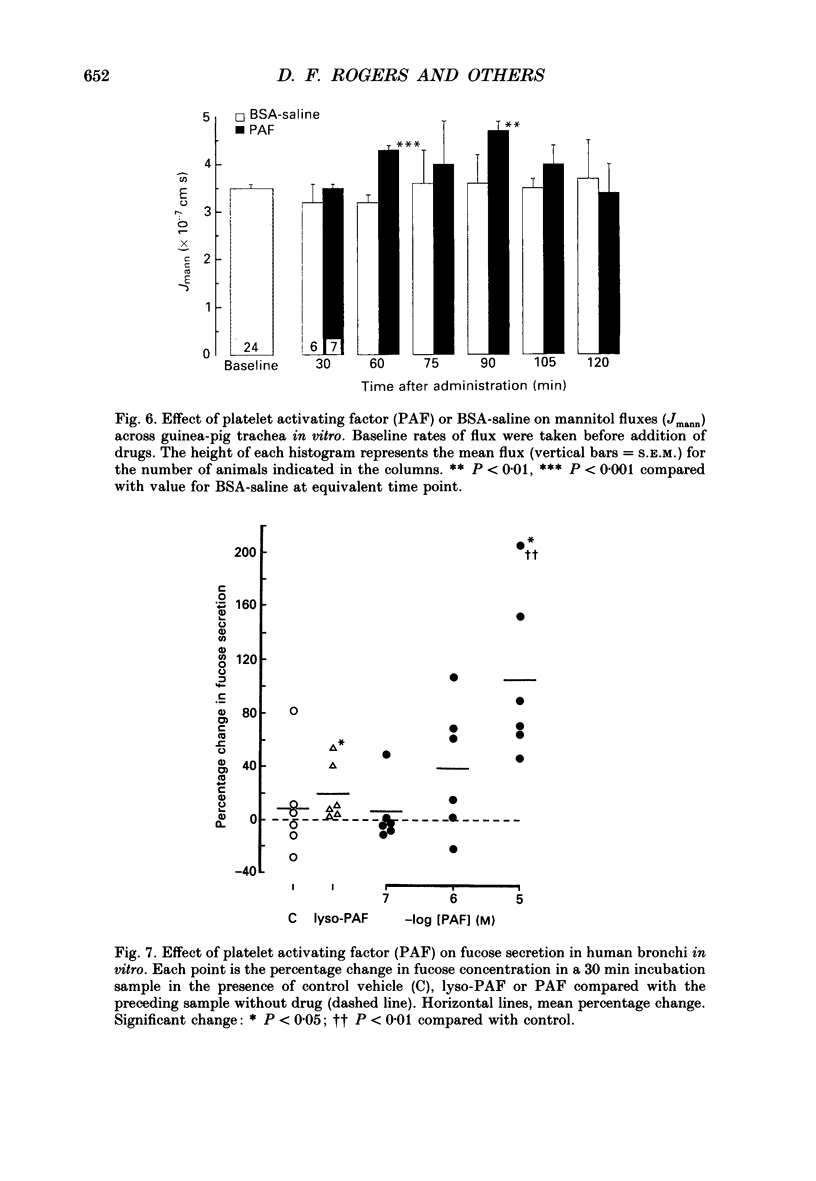






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Schwarz J. E., Anderson W. H., Welton A. F. Platelet activating factor stimulates secretion of mucin by explants of rodent airways in organ culture. Exp Lung Res. 1987;13(1):25–43. doi: 10.3109/01902148709064307. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Chung K. F., Page C. P. Inflammatory mediators and asthma. Pharmacol Rev. 1988 Mar;40(1):49–84. [PubMed] [Google Scholar]
- Boucher R. C., Jr, Bromberg P. A., Gatzy J. T. Airway transepithelial electric potential in vivo: species and regional differences. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jan;48(1):169–176. doi: 10.1152/jappl.1980.48.1.169. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Rogers D. F., Barnes P. J., Evans T. W. The role of increased airway microvascular permeability and plasma exudation in asthma. Eur Respir J. 1990 Mar;3(3):329–337. [PubMed] [Google Scholar]
- Dent G., Ukena D., Sybrecht G. W., Barnes P. J. [3H]WEB 2086 labels platelet activating factor receptors in guinea pig and human lung. Eur J Pharmacol. 1989 Oct 10;169(2-3):313–316. doi: 10.1016/0014-2999(89)90029-0. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2(2):93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Evans T. W., Chung K. F., Rogers D. F., Barnes P. J. Effect of platelet-activating factor on airway vascular permeability: possible mechanisms. J Appl Physiol (1985) 1987 Aug;63(2):479–484. doi: 10.1152/jappl.1987.63.2.479. [DOI] [PubMed] [Google Scholar]
- Hogg J. C., Eggleston P. A. Is asthma an epithelial disease? Am Rev Respir Dis. 1984 Feb;129(2):207–208. [PubMed] [Google Scholar]
- Jeffery P. K. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang M., Hansen D., Hahn H. L. Effects of the PAF-antagonist CV-3988 on PAF-induced changes in mucus secretion and in respiratory and circulatory variables in ferrets. Agents Actions Suppl. 1987;21:245–252. doi: 10.1007/978-3-0348-7451-9_22. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Morita Y., Kuriyama M., Ishihara K., Ito K., Miyamoto T. Platelet-activating factor in late asthmatic response. Int Arch Allergy Appl Immunol. 1987;82(1):57–61. doi: 10.1159/000234290. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Barnett C. J. Microvascular leakage to platelet activating factor in guinea-pig trachea and bronchi. Eur J Pharmacol. 1987 Jun 26;138(3):385–396. doi: 10.1016/0014-2999(87)90477-8. [DOI] [PubMed] [Google Scholar]
- Pack R. J., Williams I. P., Phipps R. J., Richardson P. S., Rich B. A preparation for the study of secretory function of the human bronchus in vitro. Eur J Respir Dis. 1984 May;65(4):239–250. [PubMed] [Google Scholar]
- Price A. M., Webber S. E., Widdicombe J. G. Transport of albumin by the rabbit trachea in vitro. J Appl Physiol (1985) 1990 Feb;68(2):726–730. doi: 10.1152/jappl.1990.68.2.726. [DOI] [PubMed] [Google Scholar]
- Rogers D. F., Barnes P. J. Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet. 1989 Apr 29;1(8644):930–932. doi: 10.1016/s0140-6736(89)92509-9. [DOI] [PubMed] [Google Scholar]
- Rogers D. F., Boschetto P., Barnes P. J. Plasma exudation. Correlation between Evans blue dye and radiolabeled albumin in guinea pig airways in vivo. J Pharmacol Methods. 1989 Jul;21(4):309–315. doi: 10.1016/0160-5402(89)90068-5. [DOI] [PubMed] [Google Scholar]
- Rogers D. F., Turner N. C., Marriott C., Jeffery P. K. Cigarette smoke-induced 'chronic bronchitis': a study in situ of laryngo-tracheal hypersecretion in the rat. Clin Sci (Lond) 1987 May;72(5):629–637. doi: 10.1042/cs0720629. [DOI] [PubMed] [Google Scholar]
- Steiger J., Bray M. A., Subramanian N. Platelet activating factor (PAF) is a potent stimulator of porcine tracheal fluid secretion in vitro. Eur J Pharmacol. 1987 Oct 27;142(3):367–372. doi: 10.1016/0014-2999(87)90075-6. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Schwab J. H., Chen M. G., Knowles M. R., Boucher R. C. Effects of Pseudomonas aeruginosa on bronchial epithelial ion transport. Am Rev Respir Dis. 1986 Jul;134(1):17–21. doi: 10.1164/arrd.1986.134.1.17. [DOI] [PubMed] [Google Scholar]
- Webber S. E., Widdicombe J. G. The transport of albumin across the ferret in vitro whole trachea. J Physiol. 1989 Jan;408:457–472. doi: 10.1113/jphysiol.1989.sp017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Widdicombe J. H., Nadel J. A. Fluid transport across the canine tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):905–909. doi: 10.1152/jappl.1980.49.5.905. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Widdicombe J. H. Pathways of ion movement in the canine tracheal epithelium. Am J Physiol. 1980 Sep;239(3):F215–F221. doi: 10.1152/ajprenal.1980.239.3.F215. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. G. Airway mucus. Eur Respir J. 1989 Feb;2(2):107–115. [PubMed] [Google Scholar]
- Woodward D. F., Spada C. S., Nieves A. L., Hawley S. B., Williams L. S. Platelet-activating factor causes goblet cell depletion in the conjunctiva. Eur J Pharmacol. 1989 Sep 1;168(1):23–30. doi: 10.1016/0014-2999(89)90628-6. [DOI] [PubMed] [Google Scholar]
- Yukawa T., Read R. C., Kroegel C., Rutman A., Chung K. F., Wilson R., Cole P. J., Barnes P. J. The effects of activated eosinophils and neutrophils on guinea pig airway epithelium in vitro. Am J Respir Cell Mol Biol. 1990 Apr;2(4):341–353. doi: 10.1165/ajrcmb/2.4.341. [DOI] [PubMed] [Google Scholar]


