Abstract
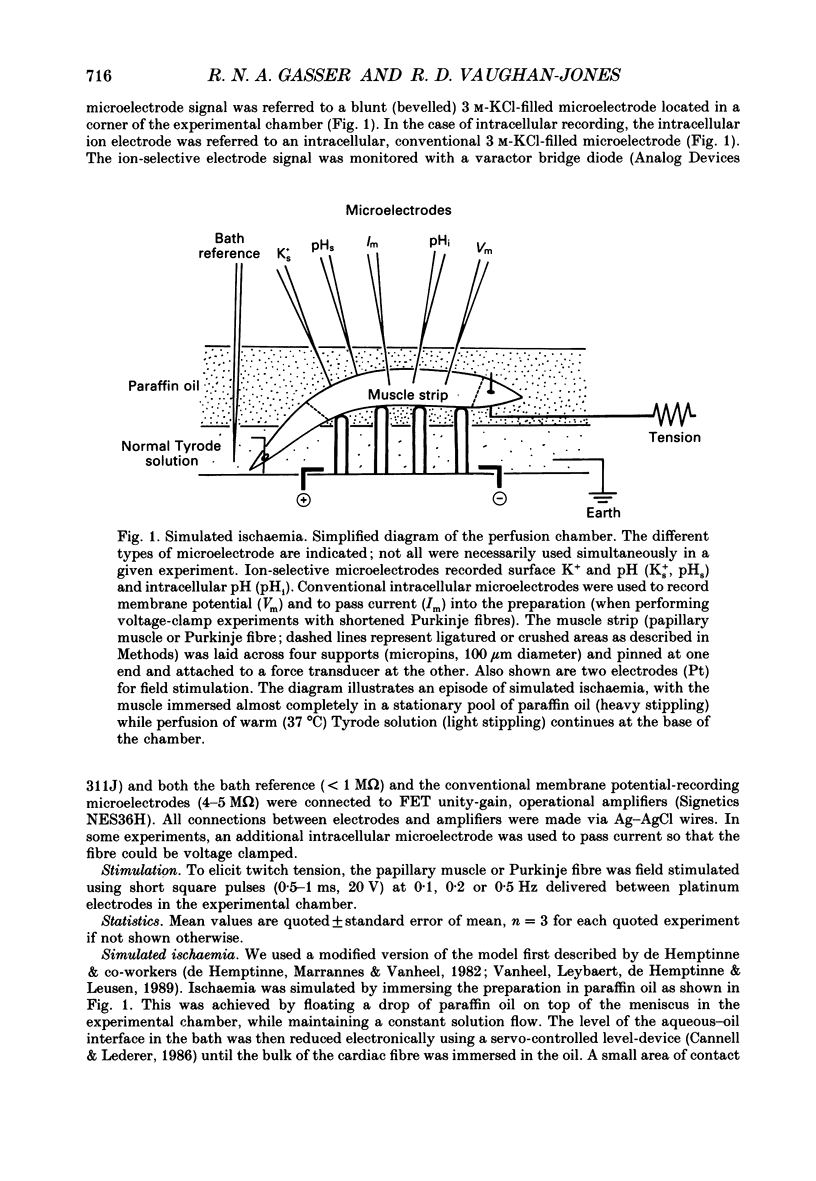
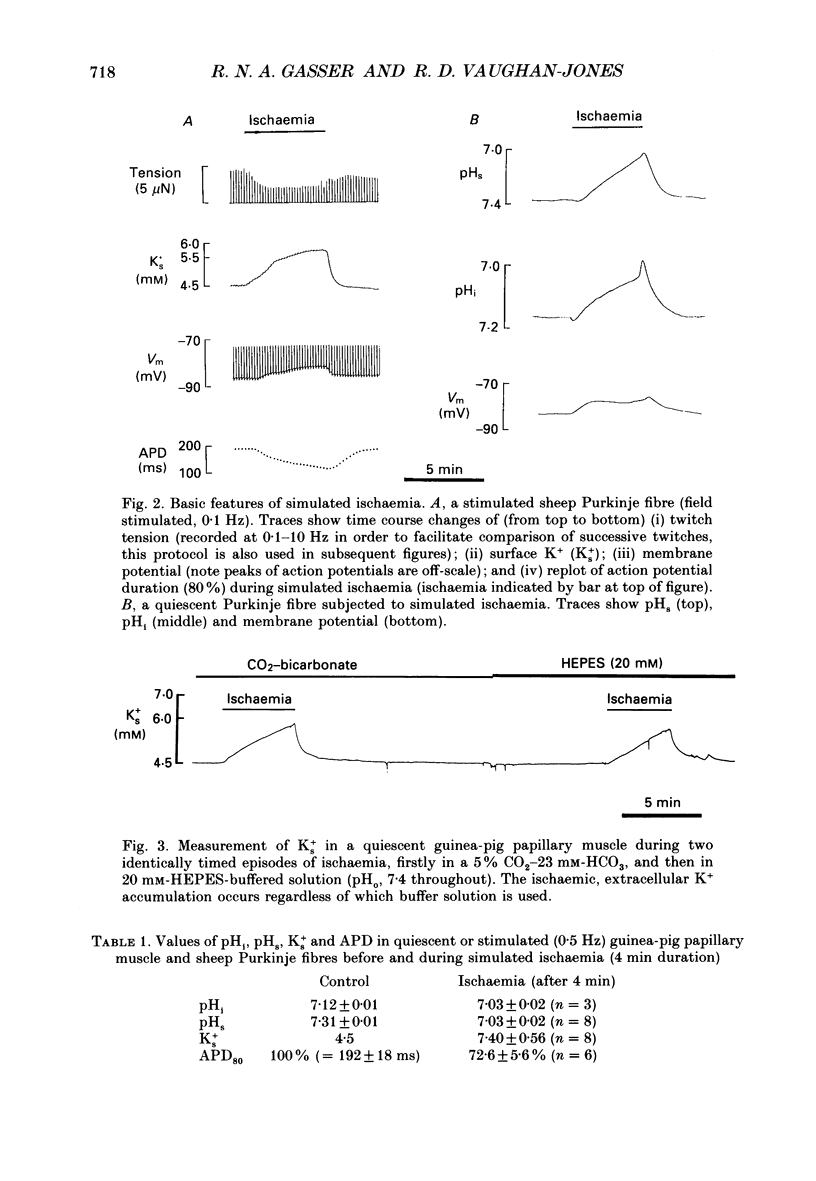
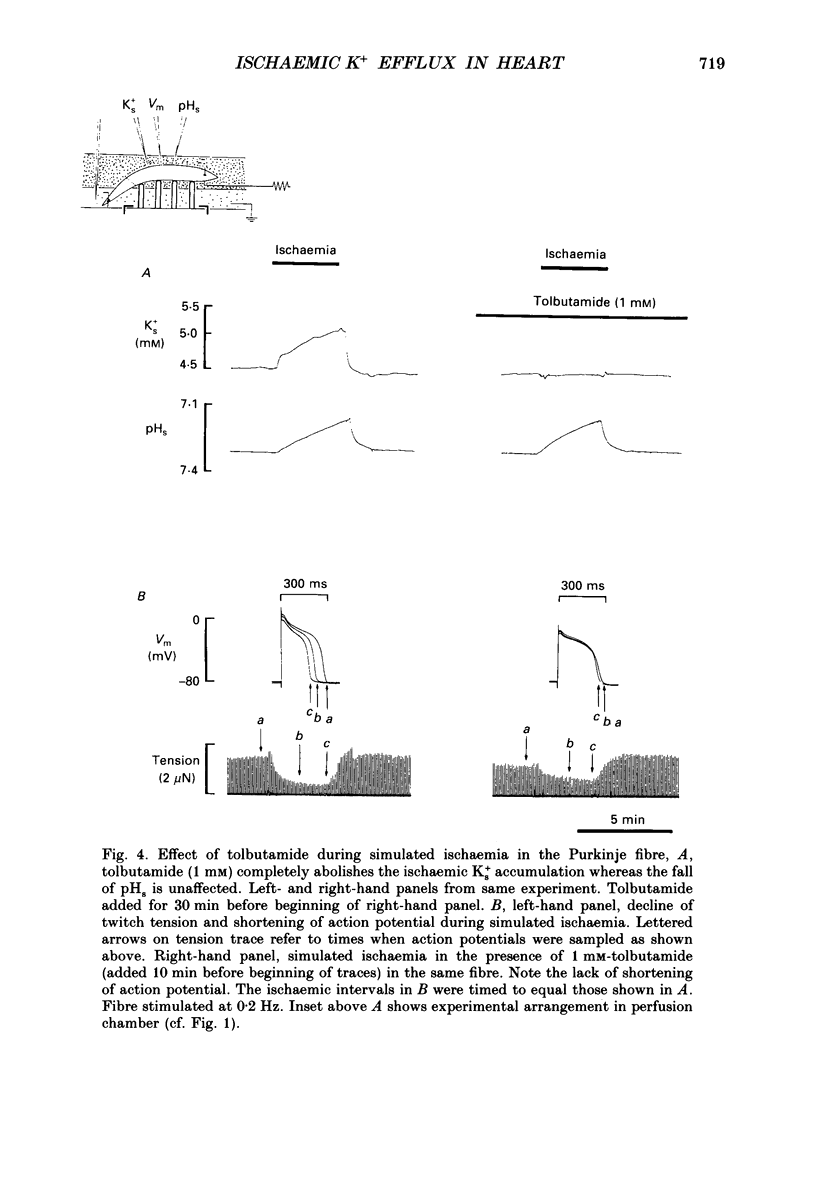
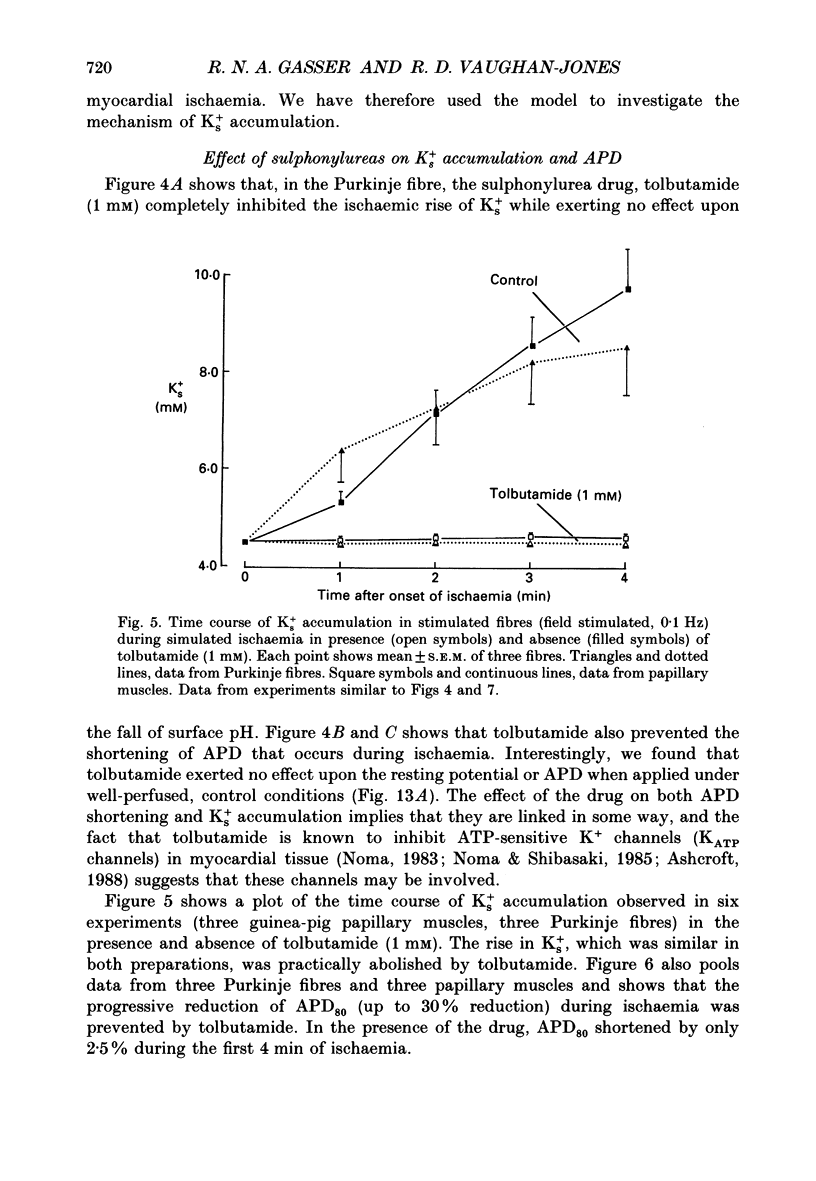
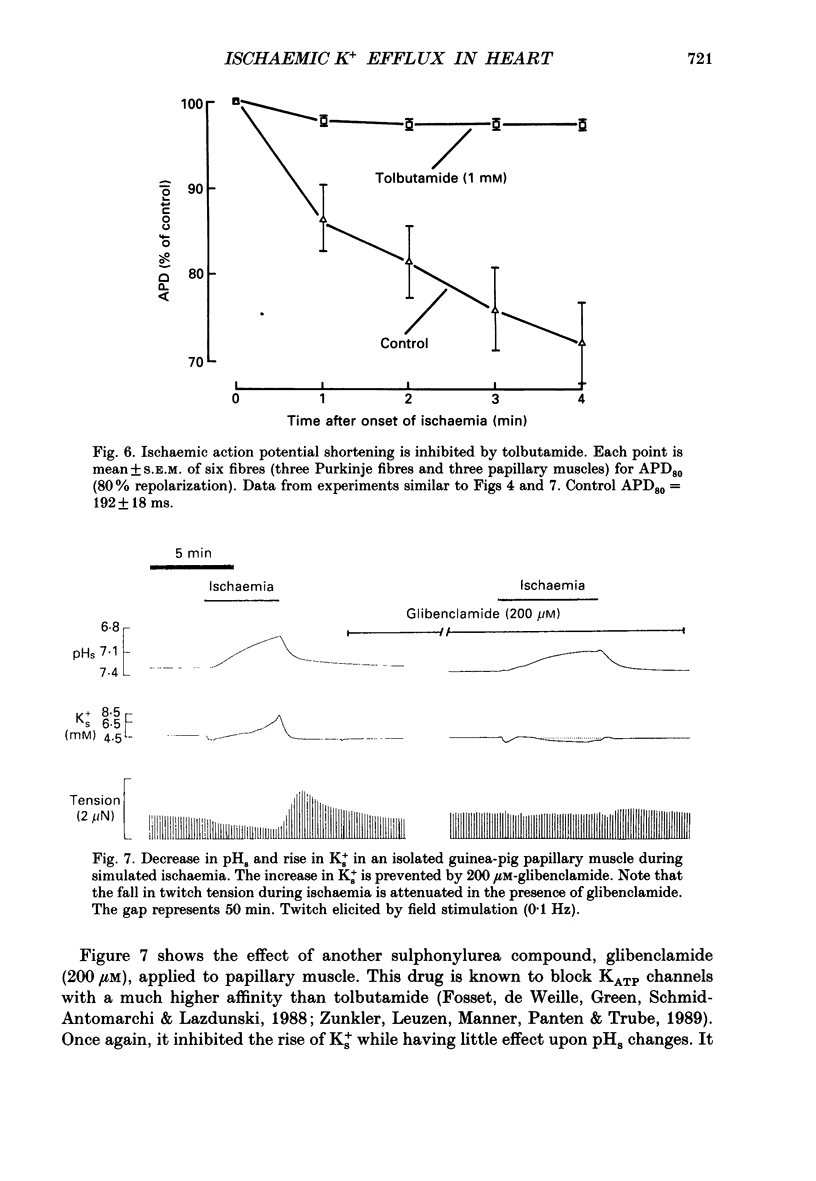
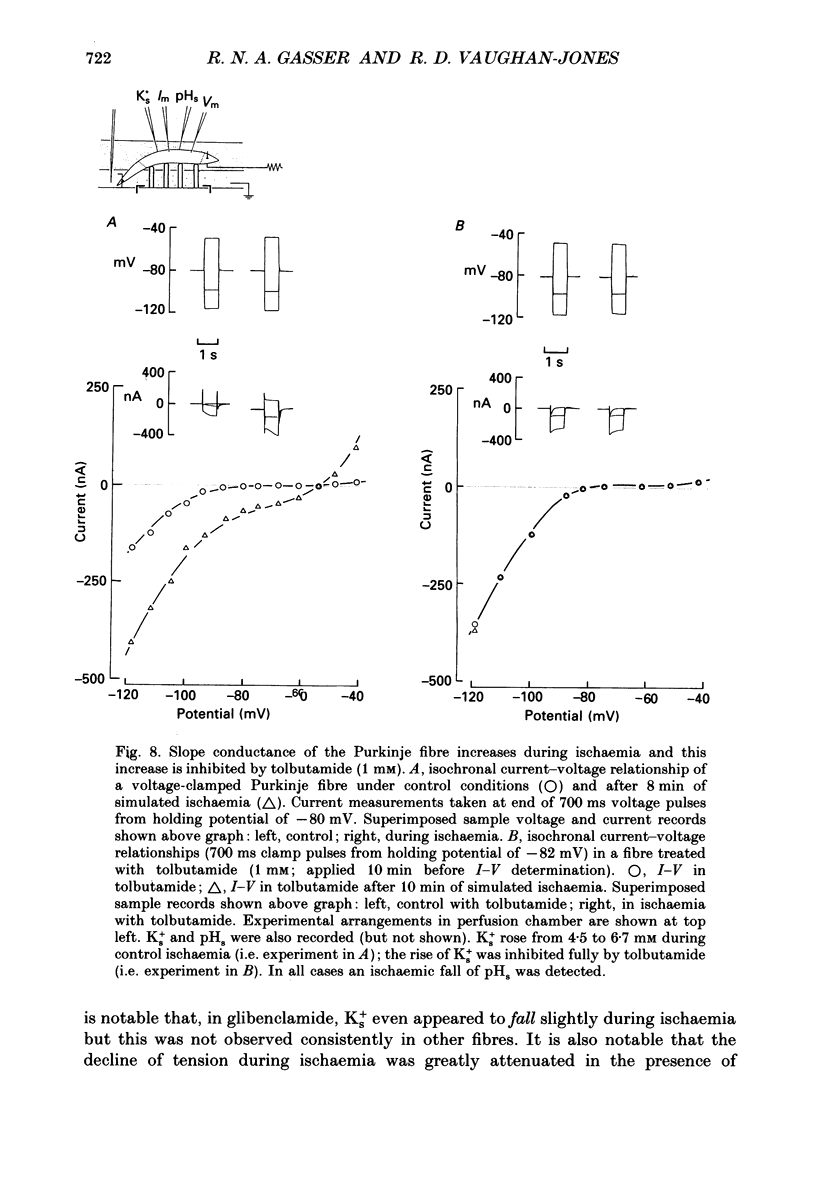
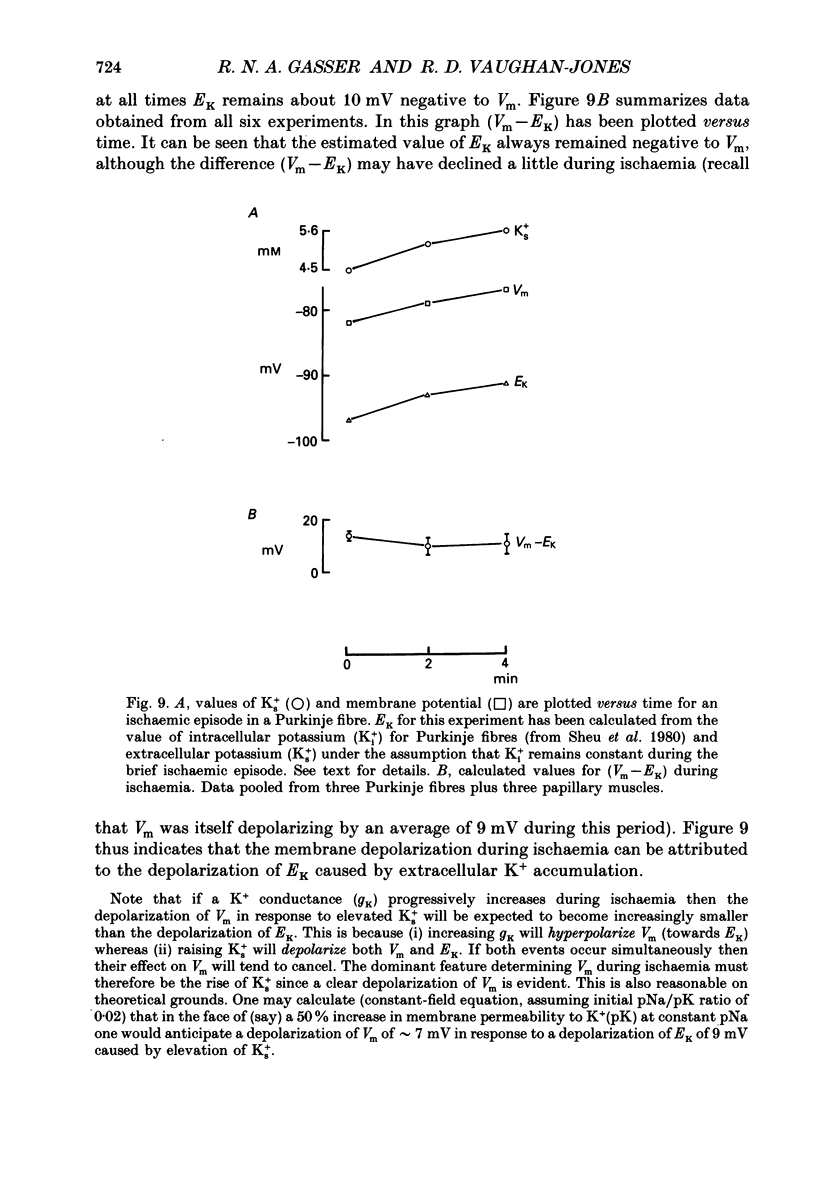
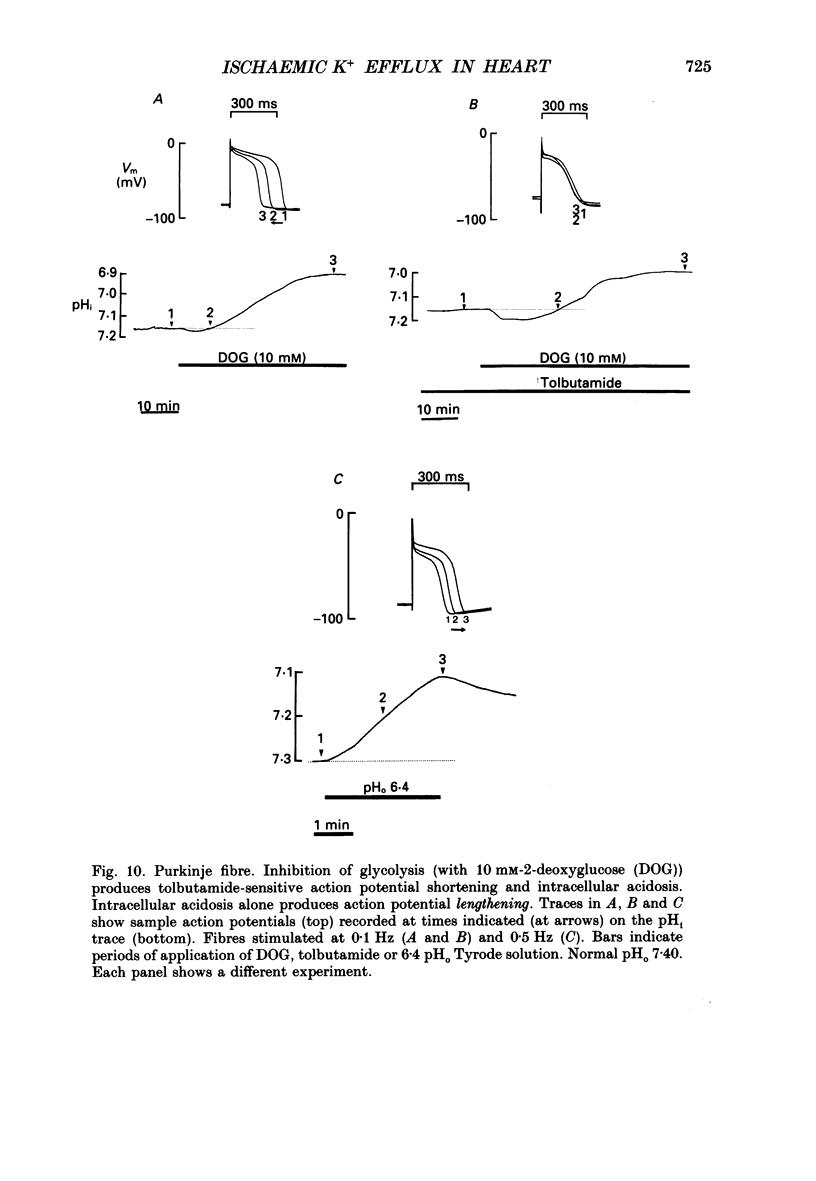
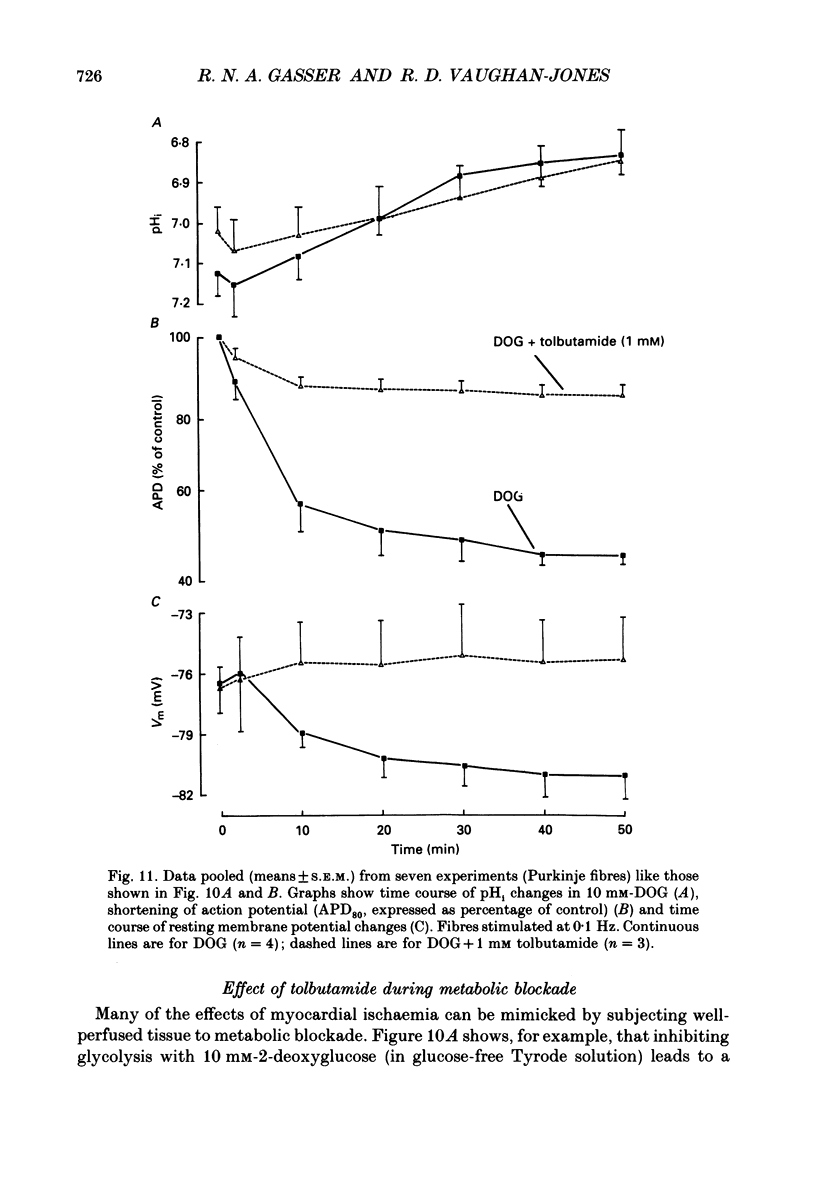
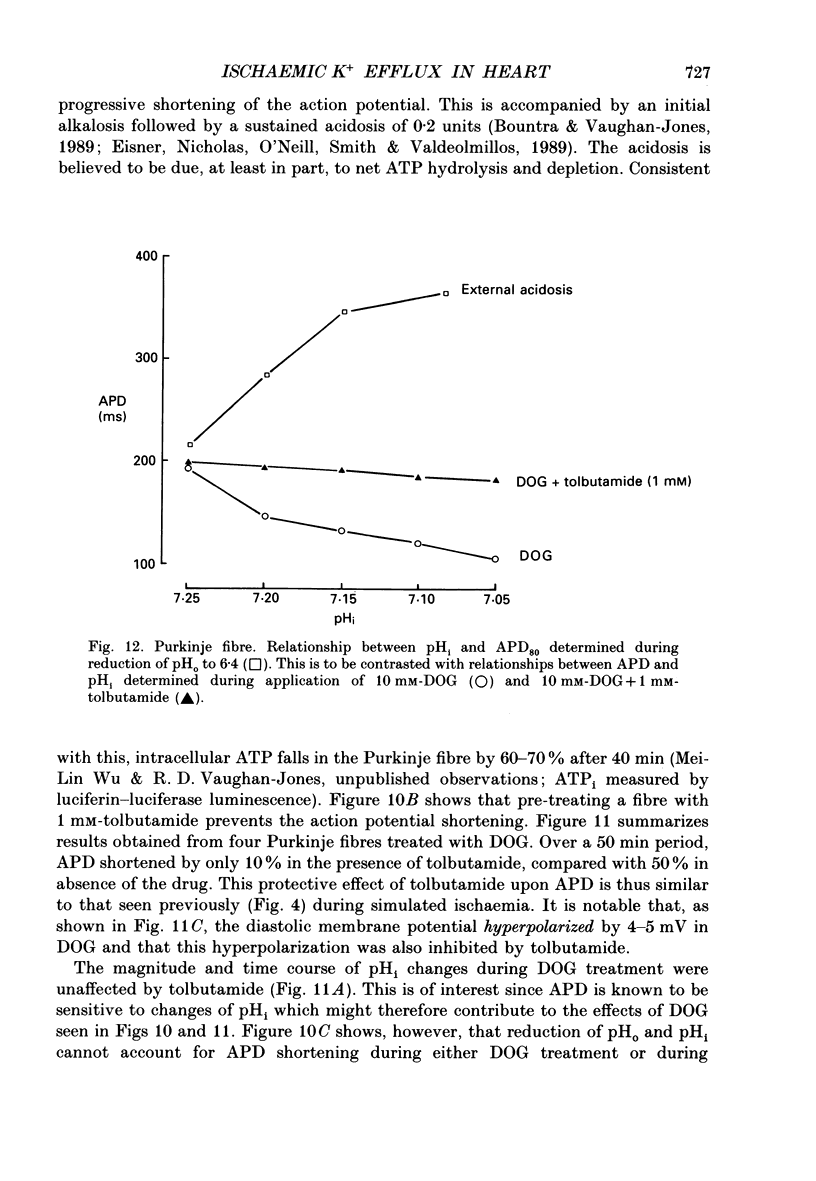
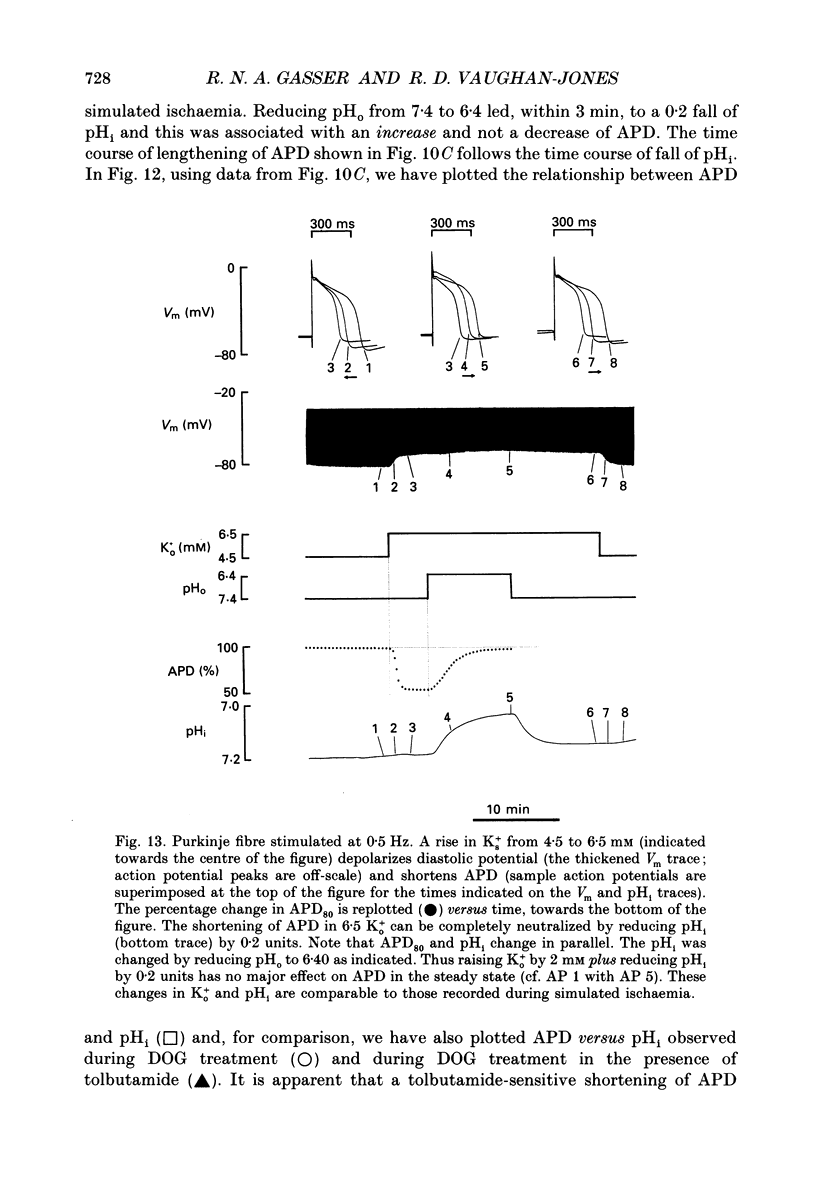
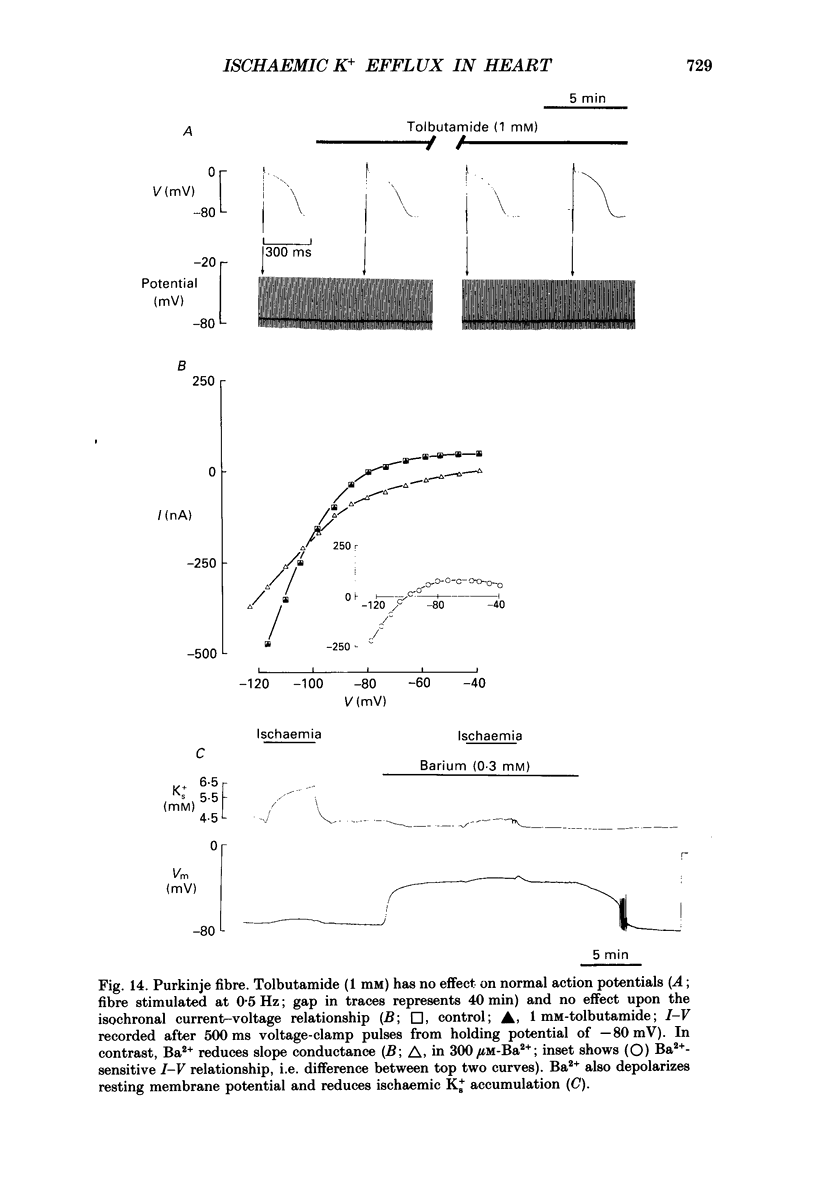
1. Ischaemia was simulated in the isolated sheep cardiac Purkinje fibre and guinea-pig papillary muscle by immersing the preparations in paraffin oil. Ion-selective microelectrodes recorded potassium (Ks+) and pH (pHs) in the thin film of Tyrode solution trapped at the fibre surface while other microelectrodes recorded intracellular pH (pHi), membrane potential and action potentials (AP) (evoked by field stimulation), or membrane current (two-microelectrode voltage clamp in shortened Purkinje fibres). Twitch tension was also monitored. The paraffin oil model reproduced the salient characteristics of myocardial ischaemia, i.e. a decrease of twitch tension; a decrease of pHi and pHs; a rise in Ks+ (by 2-3 mM); a depolarization of diastolic membrane potential; considerable shortening of the AP (up to 30% within 4 min). 2. The sulphonylurea compounds, glibenclamide (200 microM) and tolbutamide (1 mM), known inhibitors of the KATP channel, completely blocked the ischaemic rise of Ks+ and prevented AP shortening. Ischaemic tension decline was notably less pronounced in the presence of sulphonylureas. 3. The ischaemic increase of slope conductance (Purkinje fibre) was prevented by 1 mM-tolbutamide and 200 microM-glibenclamide. 4. Sulphonylureas did not affect resting membrane potential, the AP or the current-voltage relationship under non-ischaemic conditions (this also indicates that ischaemic Ks+ accumulation is not fuelled by the background K+ current [iK1] which was shown, as expected, to be Ba2+ sensitive). 5. In a normally perfused preparation, reducing intracellular ATP by inhibiting glycolysis with 2-deoxyglucose (DOG) produced a similar AP shortening plus a membrane hyperpolarization, both of which were inhibited by tolbutamide or glibenclamide. The AP shortening was not related uniquely to the fall of pHi observed under these conditions since experimentally reducing pHi (by reducing pHo in the absence of DOG) lengthened rather than shortened the AP. 6. The possibility that the ischaemic rise in Ks+ might be the cause of AP shortening was excluded by the observation that, in a normally perfused Purkinje fibre, experimentally reducing pHi (by an amount similar to that seen during ischaemia) completely neutralized the AP-shortening effect of an elevated Ko+ (from 4.5 to 6.5 mM). Furthermore, the sulphonylurea-sensitive AP shortening seen during DOG treatment could not have been associated with a Ks+ rise since, in these particular experiments, the fibres were well perfused and diastolic membrane potential hyperpolarized.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Jones D. P. ATP concentration gradients in cytosol of liver cells during hypoxia. Am J Physiol. 1985 Nov;249(5 Pt 1):C385–C392. doi: 10.1152/ajpcell.1985.249.5.C385. [DOI] [PubMed] [Google Scholar]
- Belles B., Hescheler J., Trube G. Changes of membrane currents in cardiac cells induced by long whole-cell recordings and tolbutamide. Pflugers Arch. 1987 Aug;409(6):582–588. doi: 10.1007/BF00584657. [DOI] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Jr, Cohen I., Noble D. The interactions of protons, calcium and potassium ions on cardiac Purkinje fibres. J Physiol. 1978 Sep;282:345–352. doi: 10.1113/jphysiol.1978.sp012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987 Feb;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Stewart G. W. The human erythrocyte Cl-dependent Na-K cotransport system as a possible model for studying the action of loop diuretics. Br J Pharmacol. 1982 Jan;75(1):183–188. doi: 10.1111/j.1476-5381.1982.tb08771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R., Moffat M. P., Lukas A. Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion. Studies on isolated canine ventricular tissues. Circ Res. 1985 Feb;56(2):184–194. doi: 10.1161/01.res.56.2.184. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Weille J. R., Green R. D., Schmid-Antomarchi H., Lazdunski M. Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels. J Biol Chem. 1988 Jun 15;263(17):7933–7936. [PubMed] [Google Scholar]
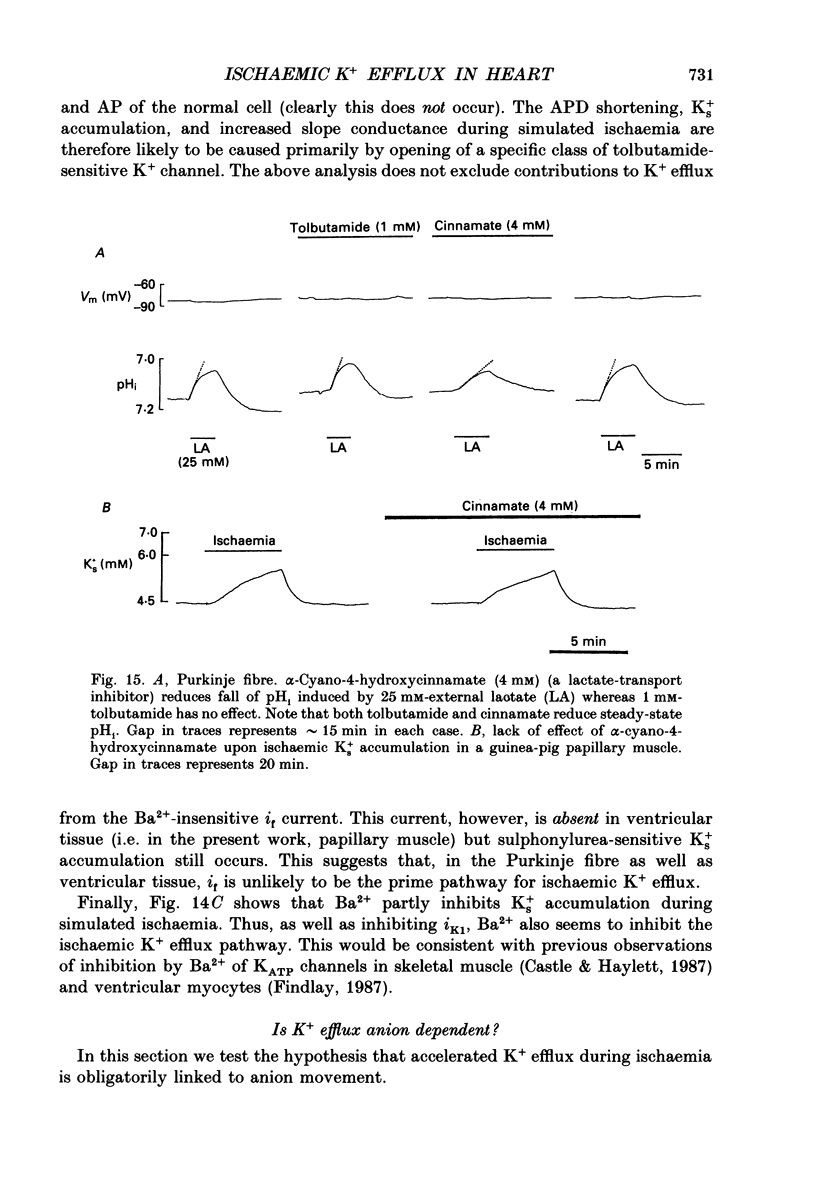
- Gaspardone A., Shine K. I., Seabrooke S. R., Poole-Wilson P. A. Potassium loss from rabbit myocardium during hypoxia: evidence for passive efflux linked to anion extrusion. J Mol Cell Cardiol. 1986 Apr;18(4):389–399. doi: 10.1016/s0022-2828(86)80902-6. [DOI] [PubMed] [Google Scholar]
- Gasser R., Dienstl F. Acute myocardial infarction: an episodic event of several coronary spasms followed by dilatation? Clin Physiol. 1986 Oct;6(5):397–403. doi: 10.1111/j.1475-097x.1986.tb00070.x. [DOI] [PubMed] [Google Scholar]
- HARRIS A. S., BISTENI A., RUSSELL R. A., BRIGHAM J. C., FIRESTONE J. E. Excitatory factors in ventricular tachycardia resulting from myocardial ischemia; potassium a major excitant. Science. 1954 Feb 12;119(3085):200–203. doi: 10.1126/science.119.3085.200. [DOI] [PubMed] [Google Scholar]
- Hackett D., Davies G., Chierchia S., Maseri A. Intermittent coronary occlusion in acute myocardial infarction. Value of combined thrombolytic and vasodilator therapy. N Engl J Med. 1987 Oct 22;317(17):1055–1059. doi: 10.1056/NEJM198710223171704. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
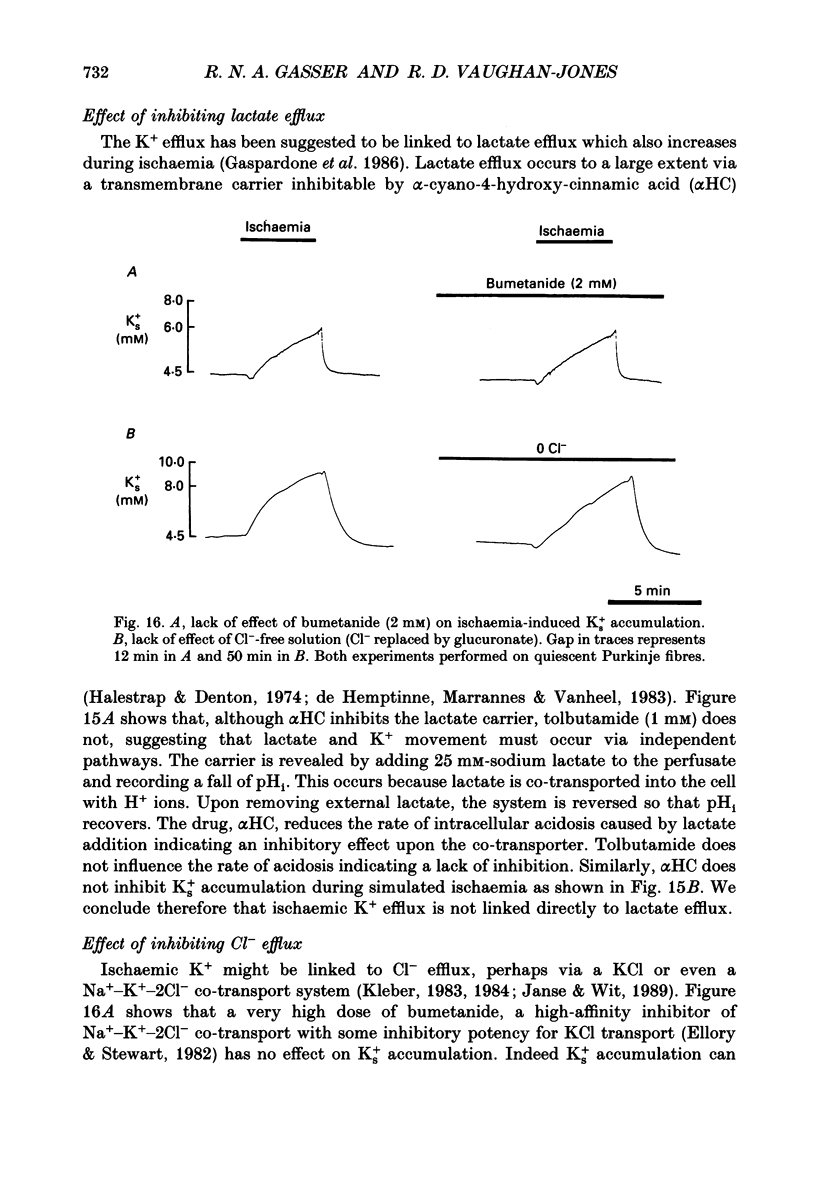
- Harris A. S. Potassium and experimental coronary occlusion. Am Heart J. 1966 Jun;71(6):797–802. doi: 10.1016/0002-8703(66)90601-6. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Vereecke J., van der Heyden G., Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983 Jun 1;397(4):251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Kantor P. F., Coetzee W. A., Carmeliet E. E., Dennis S. C., Opie L. H. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res. 1990 Feb;66(2):478–485. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Braunwald E. Observations on experimental myocardial ischaemia. Cardiovasc Res. 1980 Jul;14(7):371–395. doi: 10.1093/cvr/14.7.371. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984 May;16(5):389–394. doi: 10.1016/s0022-2828(84)80610-0. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G., Smith G. L. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989 Jun;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey G. S., Lasseter K. C., Palmer R. F. Sulfonylureas and the heart. Annu Rev Med. 1974;25:69–74. doi: 10.1146/annurev.me.25.020174.000441. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. Anoxia-recovery cycle in ventricular muscle: action potential duration, contractility and ATP content. Pflugers Arch. 1971;325(4):305–322. doi: 10.1007/BF00592172. [DOI] [PubMed] [Google Scholar]
- Miller D. S., Horowitz S. B. Intracellular compartmentalization of adenosine triphosphate. J Biol Chem. 1986 Oct 25;261(30):13911–13915. [PubMed] [Google Scholar]
- Miura D. S., Hoffman B. F., Rosen M. R. The effect of extracellular potassium on the intracellular potassium ion activity and transmembrane potentials of beating canine cardiac Purkinje fibers. J Gen Physiol. 1977 Apr;69(4):463–474. doi: 10.1085/jgp.69.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Yang Q. F., Trautwein W. Effects of barium on the membrane currents in the rabbit S-A node. Pflugers Arch. 1982 Jul;394(1):78–84. doi: 10.1007/BF01108311. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A. Potassium and the heart. Clin Endocrinol Metab. 1984 Jul;13(2):249–268. doi: 10.1016/s0300-595x(84)80021-3. [DOI] [PubMed] [Google Scholar]
- Rau E. E., Shine K. I., Langer G. A. Potassium exchange and mechanical performance in anoxic mammalian myocardium. Am J Physiol. 1977 Jan;232(1):H85–H94. doi: 10.1152/ajpheart.1977.232.1.H85. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Korth M., Lathrop D. A., Fozzard H. A. Intra- and extracellular K+ and Na+ activities and resting membrane potential in sheep cardiac purkinje strands. Circ Res. 1980 Nov;47(5):692–700. doi: 10.1161/01.res.47.5.692. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Chien A. M., Capogrossi M. C., Pelto D. J., Lakatta E. G. Direct observation of the "oxygen paradox" in single rat ventricular myocytes. Circ Res. 1985 Jun;56(6):899–903. doi: 10.1161/01.res.56.6.899. [DOI] [PubMed] [Google Scholar]
- Vanheel B., Leybaert L., De Hemptinne A., Leusen I. Simulated ischemia and intracellular pH in isolated ventricular muscle. Am J Physiol. 1989 Aug;257(2 Pt 1):C365–C376. doi: 10.1152/ajpcell.1989.257.2.C365. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Kaila K. The sensitivity of liquid sensor, ion-selective microelectrodes to changes in temperature and solution level. Pflugers Arch. 1986 Jun;406(6):641–644. doi: 10.1007/BF00584033. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugels A., Vereecke J., Carmeliet E. Ionic currents during hypoxia in voltage-clamped cat ventricular muscle. Circ Res. 1980 Oct;47(4):501–508. doi: 10.1161/01.res.47.4.501. [DOI] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. Extracellular K+ accumulation during myocardial ischemia in isolated rabbit heart. Am J Physiol. 1982 Apr;242(4):H619–H628. doi: 10.1152/ajpheart.1982.242.4.H619. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Influence of organic acids on intracellular pH. Am J Physiol. 1983 Sep;245(3):C178–C183. doi: 10.1152/ajpcell.1983.245.3.C178. [DOI] [PubMed] [Google Scholar]


