Abstract
Hereditary paraganglioma syndrome has recently been shown to be caused by germline heterozygous mutations in three (SDHB, SDHC, and SDHD) of the four genes that encode mitochondrial succinate dehydrogenase. Extraparaganglial component neoplasias have never been previously documented. In a population-based registry of symptomatic presentations of phaeochromocytoma/paraganglioma comprising 352 registrants, among whom 16 unrelated registrants were SDHB mutation positive, one family with germline SDHB mutation c.847-50delTCTC had two members with renal cell carcinoma (RCC), of solid histology, at ages 24 and 26 years. Both also had paraganglioma. A registry of early-onset RCCs revealed a family comprising a son with clear-cell RCC and his mother with a cardiac tumor, both with the germline SDHB R27X mutation. The cardiac tumor proved to be a paraganglioma. All RCCs showed loss of the remaining wild-type allele. Our observations suggest that germline SDHB mutations can predispose to early-onset kidney cancers in addition to paragangliomas and carry implications for medical surveillance.
Hereditary neoplasia syndromes that include pheochromocytomas or extra-adrenal pheochromocytomas (paragangliomas [PGLs]) included multiple-endocrine neoplasia type 2 (MEN 2 [MIM 171400]), von Hippel-Lindau disease (VHL [MIM 193300]), and type 1 neurofibromatosis (MIM 162200) for several decades. Recently, germline mutations in SDHD (MIM 602690), a nuclear gene encoding the D subunit of the mitochondrial enzyme succinate dehydrogenase (SDH), were described in families with head and neck PGLs (Baysal et al. 2000). Subsequently, the tumor spectrum for germline mutations in SDHD was expanded to include familial and apparently sporadic pheochromocytomas (reviewed by Maher and Eng [2002]). It is interesting that germline mutations in genes encoding two other subunits of SDH (mitochondrial complex II), SDHB (MIM 185470 and MIM 605373) and SDHC (MIM 602413), were found to be associated with heritable pheochromocytoma and/or PGL (Maher and Eng 2002; Eng et al. 2003). No germline mutations in SDHA (MIM 600857) have been found in pheochromocytoma or PGL cases. Instead, homozygous SDHA mutations result in congenital severe neurodegeneration and seizures. In a population-based study, ∼25% of all apparently sporadic (defined as “without syndromic features and family history”) clinical presentations of pheochromocytoma were found to harbor occult germline mutations in SDHB, SDHD, VHL (which results in VHL), and RET (which results in MEN 2) (Neumann et al. 2002). Until now, it was believed that there were no other extraparaganglial manifestations in those carrying SDH mutations (Baysal 2002; Baysal et al. 2002; Maher and Eng 2002; Eng et al. 2003).
Apart from SDH, germline heterozygous mutations in only one other nuclear-encoded gene that codes for a mitochondrial enzyme, FH-encoding fumarate hydratase (fumarase [MIM 136850]), has been described in a seemingly unrelated inherited cancer syndrome, hereditary leiomyomatosis and renal cell carcinoma (HLRCC [MIM 605839]) (Tomlinson et al. 2002). Paralleling SDH, homozygous germline mutations in FH cause severe neurodegeneration (FH deficiency [MIM 606812]) (Eng et al. 2003). Therefore, because of the mitochondrial etiologies for both SDH-related heritable PGL and HLRCC, we sought to determine the existence of PGL in a population-based registry of renal cell carcinoma (RCC) and the existence of RCC in a population-based registry of symptomatic presentations of PGL and adrenal pheochromocytomas.
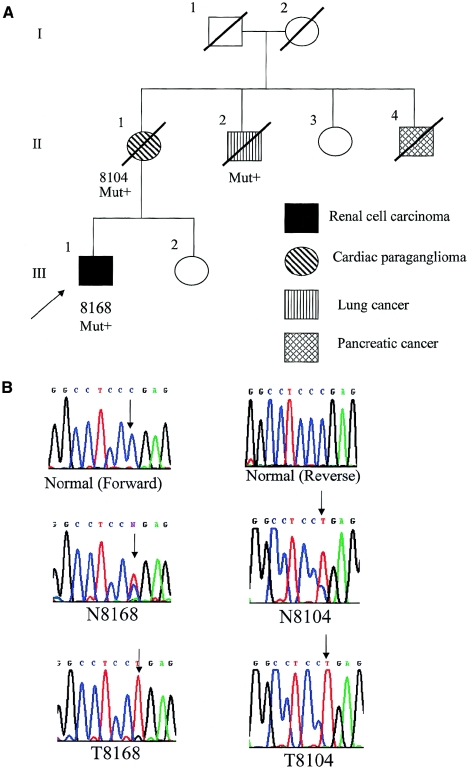
Family 1 was ascertained in a computerized search of the national Finnish Cancer Registry for RCC cases diagnosed at ages 15–34 years, followed by construction of pedigree and documentation through patient records, in accordance with the institutional review boards for human subjects protection of the University of Helsinki and The Ohio State University. This search revealed 244 unrelated patients with RCC. After pedigree expansion and documentation, family 1 was ascertained. The index patient received the diagnosis of RCC at age 28 years (fig. 1A). At the time of diagnosis, the tumor had already widely metastasized, and a palliative nephrectomy was performed. The resected kidney, encompassing the tumor, weighed 2,915 g and measured 17 × 23 cm. Histologically, the tumor was a conventional (clear cell) carcinoma that showed a mixture of clear cells and cells with granular-eosinophilic cytoplasm. The proband’s mother (patient II-1) (fig. 1A), at age 55 years, received a diagnosis of a malignant PGL of the heart growing from the septum to the right ventricle. In her case, the tumor was considered inoperable, and it eventually led to the demise of the patient. The proband’s maternal uncles (patients II-2 and II-4) had small-cell lung carcinoma, diagnosed at age 55 years, and pancreatic carcinoma, diagnosed at age 71 years, respectively (fig. 1). No samples from individual II-4 were available for further analysis.
Figure 1.
Family 1, with documented RCC, cardiac PGL, and the germline SDHB R27X mutation. A, Pedigree of family 1. Generation numbers are represented by Roman numerals. Individual numbers are in Arabic numerals. The index patient (proband) is III-1, indicated by the arrow. “Mut+” indicates mutation-positive individuals. B, Sequencing chromatogram representing part of SDHB exon 2. The sequences around codon 27 from a normal control is shown at the top (“Normal”). The germline of the proband showed a heterozygous R27X mutation with a wild-type C and a mutant T (N8168). The tumor DNA shows loss of the wild-type C allele, leaving only the mutant T (T8168). The proband’s mother’s germline (N8104) showed the heterozygous R27X mutation, whereas her tumor (T8104) showed loss of the remaining wild-type allele. (Note that, although the right panel was obtained in reverse sequence, the reverse complement chromatogram, which represents a computer-generated forward sequence, is shown for ease of viewing.)
Germline genomic DNA from archived normal tissue of the deceased index patient was examined for the presence of constitutional mutations in SDHA, SDHB, SDHC, and SDHD by direct semiautomated sequence analysis, as described elsewhere (Neumann et al. 2002) (primers for SDHA analysis available on request from C.E. [eng.25@osu.edu]). A germline heterozygous truncating mutation in SDHB, R27X, was found in the proband (III-1), his mother (II-1), and his uncle (II-2). DNA was then extracted from the proband’s RCC, his mother’s cardiac PGL, and his uncle’s lung tumor. Direct sequencing revealed somatic loss of the remaining wild-type SDHB allele in the proband’s renal tumor and in his mother’s PGL (fig. 1B) but not in his uncle’s lung carcinoma. The uncle smoked cigarettes for >40 years, and, in the absence of loss of heterozygosity of the wild-type allele, the lung cancer is probably unrelated to the SDHB mutation.
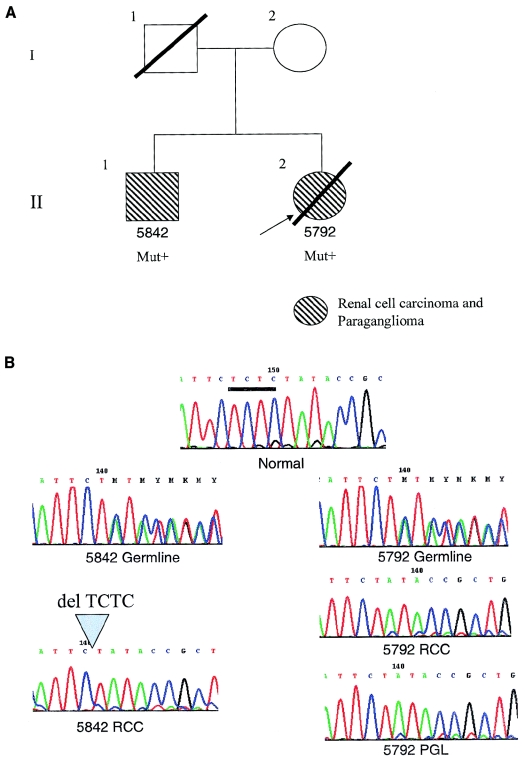
Family 2 was ascertained from a population-based registry of the Freiburg-Warsaw-Columbus Phaeochromocytoma Study Group, which registers all clinical presentations of pheochromocytomas in Germany and central Poland, in accordance with the institutional review boards of The Ohio State University, the University of Freiburg, and the Institute of Cardiology, Warsaw (Neumann et al. 2002) (fig. 2A). In this registry, there are 352 unrelated registrants, and 16 (∼5%) have been found to carry germline SDHB mutations (Neumann et al. 2002; H. Neumann and C. Eng, unpublished observations). Among those 16, two siblings (patients II-2 and II-1) were found to have RCC with solid histology diagnosed at ages 24 years and 26 years, respectively (fig. 2A). Both siblings also had PGL. It is interesting that patient II-1 received a diagnosis of RCC first, before his PGL diagnosis. Direct sequencing of genomic DNA, extracted from blood leukocytes, revealed a germline heterozygous frameshift mutation in SDHB, c.847-50delTCTC. The PGL from patient II-2 and both renal tumors showed somatic loss of the remaining wild-type allele (fig. 2B).
Figure 2.
Family 2, with documented PGL and RCC and germline heterozygous SDHB c.847-50delTCTC mutation. A, Pedigree of family 2. The mutation-positive individuals are indicated by “Mut+.” The index patient is II-2 (5792), indicated by the arrow. B, Sequencing chromatogram in the region of nucleotides 845–855 of SDHB. The wild-type sequence from a normal control is in the top panel (“Normal”). The bar below the TCTC denotes the region of deletion. The germline DNA from both the index patient and her brother showed the heterozygous c.847-50delTCTC microdeletion mutation. Note that, because of the nature of the repeats, we cannot ascertain whether the microdeletion is c.847-50delTCTC or c.848-51delCTCT. When the mutant allele is present heterozygously with the wild type (“5842 germline” and “5792 germline”), the reading frame is shifted at the point of microdeletion. When the wild-type allele is lost, as in all three tumors (“5842 RCC,” “5792 RCC,” and “5792 PGL”), the mutant allele remains.
To determine whether SDH also plays a role in all individuals with generic sporadic renal carcinoma, we examined SDHA, SDHB, SDHC, and SDHD for germline and somatic mutations in 60 sporadic RCCs (30 clear cell, 3 papillary, 2 granular cell, 1 mixed papillary/clear cell, 1 mixed clear cell/solid histology, 9 oncocytic papillary, and 14 oncocytoma) diagnosed at any age. No germline or somatic mutations were found in these four genes. Because the SDHB-related RCCs were diagnosed at ages <30 years, we then examined for mutations in these four genes in 35 tumors with clear cell histology diagnosed at ages <50, but no mutations were found. Our SDHB-mutation–positive carriers with RCCs are particularly young (<30 years), and the common clear cell histology does not predominate in these (or among SDHB-related) RCCs. Thus, it would not be a surprise to find no mutations in our series of kidney carcinomas for which the predominant age at onset is approximately in the 60s and for which clear cell histology predominates.
To our knowledge, this is the first time that a gene encoding one subunit of the mitochondrial SDH complex has been implicated in renal carcinogenesis and the first time that extraparaganglial disease has been shown to be part of the PGL syndromes characterized by SDH mutations. SDH is a part of the Krebs tricarboxylic acid cycle and the mitochondrial electron transport chain, which are required for the energy metabolism of all cells (reviewed by Eng et al. [2003]). Anchored by SDHC and SDHD, the catalytic subunits of complex II, SDHA and SDHB, convert succinate to fumarate in an energy-dependent reaction and pass fumarate to the next enzyme in the Krebs cycle, FH. It is interesting that germline FH mutations are associated with HLRCC (Tomlinson et al. 2002). The RCCs in HLRCC typically have type II papillary histology. Nonetheless, the renal cancers seen in our families with the SDHB-positive mutation are of varied histology, ranging from solid to clear cell and cells with granular-eosinophilic cytoplasm. All these carcinomas originate from epithelial cells of the proximal renal tubule. Thus, it is etiologically interesting that a defect in mitochondrial enzymes is involved in the pathogenesis of these subhistologies of RCC. The mechanism leading to neoplastic transformation after damage to the Krebs cycle and mitochondrial electron transport is still far from fully understood, but a hypothesis that these events lead to a proliferative hypoxic signal or oxidative DNA damage has been proposed (Jeffers et al. 1997; Eng et al. 2003). Accumulation of reactive oxygen species could result from dysfunction in mitochondrial energy metabolism; results supporting this theory have been reported (Jeffers et al. 1997; Eng et al. 2003). Another possible and not mutually exclusive mechanism by which mitochondrial dysfunction may lead to neoplasia is through the role of mitochondria in apoptosis (Eng et al. 2003). Because RCCs are referred to as “oncocytic” (i.e., replete with mitochondria), the role of SDH in RCC is plausible.
Other RCC-susceptibility genes do not encode mitochondrial enzymes. Although seemingly disparate, the majority of (or perhaps all) heritable RCC syndromes might involve the HIF-VEGF pathway. VHL, caused by germline mutations in VHL, is characterized by clear cell RCC, pheochromocytoma mainly of the adrenal medulla, and hemangiomas of the CNS and retina (Latif et al. 1993; Clifford and Maher 2001; Maher and Eng 2002). A genotype-phenotype correlation exists in regard to the frequencies of RCC and pheochromocytoma in a particular family (Crossey et al. 1994; Chen et al. 1995). Truncating germline mutations are associated with RCC, whereas missense mutations are associated with pheochromocytoma (Crossey et al. 1994; Chen et al. 1995). The VHL protein has several functions; for example, binding and shepherding one of the subunits of hypoxia-inducible factor (HIF) toward ubiquitin-mediated proteolysis in the presence of oxygen (Ohh et al. 2000; Clifford and Maher 2001; Clifford et al. 2001). When VHL is nonfunctional, HIF is upregulated. When mitochondrial function is impaired (e.g., as a consequence of SDHB mutations), severe energy deficits occur and oxygen free radicals are generated. When mitochondria sense the presence of oxygen free radicals (hypoxia), HIFs are activated and are translocated to the nucleus to induce gene expression (Eng et al. 2003). Thus, it may be postulated that both RCC and pheochromocytoma/PGL susceptibility resulting from SDHB deficiency could be mechanistically related to RCC and to pheochromocytoma susceptibility secondary to VHL dysfunction, via HIF (Eng et al. 2003).
Tuberous sclerosis (TSC [MIM 191100]), associated with germline mutations in TSC1 or TSC2, is characterized by renal angiomyolipomas and cysts; dermatologic lesions, such as ash leaf patches and shagreen patches; epilepsy; mental retardation; and hamartomas of the eye (Kwiatkowski 2003). Multifocal RCCs have been reported in several TSC cases (Sampson et al. 1995; Takahashi et al. 2002). Recently, loss or dysfunction of Tsc2 in the Eker rat model was shown to result in upregulation of Hif2-α in RCC (Liu et al. 2003). Familial papillary RCC (MIM 164860) is a rare syndrome caused by germline mutations in the MET proto-oncogene (Schmidt et al. 1997). The ligand for MET, hepatocyte growth factor, has been shown to upregulate HIF-1 activity (Tacchini et al. 2001; Matteucci et al. 2003). Germline mutations in HRPT2 have been recently associated with hyperparathyroidism jaw tumor syndrome (MIM 145001), and germline mutations in BHD, encoding folliculin, have been associated with Birt-Hogg-Dubé syndrome (MIM 135150); both of these diseases have RCC as component tumors (Khoo et al. 2002; Nickerson et al. 2002). Although the function of folliculin and HRPT2 has yet to be elucidated, it would appear that the HIF-VEGF pathway might be an important downstream common pathway for renal neoplasia in many heritable RCC syndromes.
The German-Polish Registry includes 16 unrelated probands with germline SDHB mutations and a total of 31 mutation carriers (Neumann et al. 2002; H. Neumann and C. Eng, unpublished observations). Using the Registry to help estimate the frequency of RCC among SDHB mutation–positive individuals, we approximate a 5%–10% prevalence. Given the age distribution of SDHB carriers in the registry (mean age 30 years; 40% <20 years, 15% >40), this 5%–10% estimate might be slightly low. In contrast, results of the Finnish Registry search suggest a prevalence of <1% of all early-onset RCC. If our observations can be independently confirmed, then individuals and families with germline SDHB mutations should also undergo routine clinical surveillance for the development of early-onset renal carcinomas. Conversely, if a patient with RCC were found to harbor the germline SDHB mutation, then annual surveillance for pheochromocytoma and PGL should be considered. On the basis of our observations, very early–onset RCC with unusual histology (e.g., solid) should alert a clinician to take an extended family history. Like most heritable RCCs, (e.g., VHL-related [Neumann et al. 1998]), it is possible that SDHB-related RCC might also have a better prognosis. Of the three patients studied in this report, one is still alive after long follow-up; one has died of metastatic disease, but not until 6 years after diagnosis; and the third died of metastatic disease 1 year and 4 mo after initial diagnosis. Thus, longer follow-up and study of other cases are required to investigate this aspect of the disease.
Acknowledgments
We are deeply grateful to the patients who continue to participate in our research. This work was partially funded by National Institutes of Health grants R01HD39058 and R01HD39058-02S1 (to C.E.), Deutsche Forshungsgemeinschaft grant NE 571/4-1 (to H.P.H.N), and by the Finnish Cancer Society, the Sigrid Juselius Foundation, and Academy of Finland’s Center of Excellence Program project 44870 (support to L.A.A.). C.E. is the recipient of a Doris Duke Distinguished Clinical Scientist award.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MEN 2, VHL, type 1 neurofibromatosis, SDHD, pheochromocytomas, SDHB, SDHC, SDHA, fumarase, HLRCC, FH deficiency, TSC, familial papillary RCC, hyperparathyroidism jaw tumor syndrome, and Birt-Hogg-Dubé syndrome)
References
- Baysal BE (2002) Hereditary paraganglioma targets diverse paranglia. J Med Genet 39:617–622 10.1136/jmg.39.9.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW 3rd, Cornelisse CJ, Devilee P, Devlin B (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287:848–851 10.1126/science.287.5454.848 [DOI] [PubMed] [Google Scholar]
- Baysal BE, Willett-Brozick JE, Lawrence EC, Drovdlic CM, Savul SA, McLeod DR, Yee HA, Brackmann DE, Slattery WH III, Myers EN, Ferrell RE, Rubinstein WS (2002) Prevalence of SDHB, SDHC and SDHD in clinic patients with head and neck paragangliomas. J Med Genet 39:178–183 10.1136/jmg.39.3.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Kishida T, Yao M, Hustad T, Glavac D, Dean M, Gnarra JR, Orcutt ML, Duh FM, Glenn G, Green J, Hsia EY, Lamiell J, Li H, Wei MH, Schmidt L, Tory K, Kuzmin I, Stackhouse T, Latif F, Linehan M, Lerman M, Zbar B (1995) Germline mutations in the Von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat 5:66–75 [DOI] [PubMed] [Google Scholar]
- Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER (2001) Contrasting effects on HIF-1α regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet 10:1029–1038 10.1093/hmg/10.10.1029 [DOI] [PubMed] [Google Scholar]
- Clifford SC, Maher ER (2001) Von Hippel-Lindau disease: clinical and molecular perspectives. Adv Cancer Res 82:85–105 [DOI] [PubMed] [Google Scholar]
- Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER (1994) Identification of intragenic mutations in the Von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet 3:1303–1308 [DOI] [PubMed] [Google Scholar]
- Eng C, Kiuru M, Fernandez MJ, Aaltonen LA (2003) A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer 3:193–202 10.1038/nrc1013 [DOI] [PubMed] [Google Scholar]
- Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, vande Woude GF (1997) Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA 94:11445–11450 10.1073/pnas.94.21.11445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo SK, Giraud S, Kahnoski K, Chen J, Motoma O, Nickolov R, Binet O, Lambert D, Friedel J, Levy R, Ferlicot S, Wolkenstein P, Hammel P, Bergerheim U, Hedblad MA, Bradley M, Teh BT, Nordenskjold M, Richard S (2002) Clinical and genetic studies of Birt-Hogg-Dubé syndrome. J Med Genet 39:906–912 10.1136/jmg.39.12.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ (2003) Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet 67:87–96 10.1046/j.1469-1809.2003.00012.x [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh F-M, Orcutt M-L, Stackhouse T, et al (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260:1317–1320 [DOI] [PubMed] [Google Scholar]
- Liu MY, Poellinger L, Walker CL (2003) Up-regulation of hypoxia-inducible factor 2α in renal cell carcinoma associated with loss of Tsc-2 tumor suppressor gene. Cancer Res 15:2675–2680 [PubMed] [Google Scholar]
- Maher ER, Eng C (2002) The pressure rises: update on the genetics of phaeochromocytoma. Hum Mol Genet 11:2347–2354 10.1093/hmg/11.20.2347 [DOI] [PubMed] [Google Scholar]
- Matteucci E, Modora S, Simone M, Desiderio MA (2003) Hepatocyte growth factor induces apoptosis through the extrinsic pathway in hepatoma cells. Oncogene 22:4062–4073 10.1038/sj.onc.1206519 [DOI] [PubMed] [Google Scholar]
- Neumann HPH, Bender BU, Berger DP, Laubenberger J, Schultze-Seeman W, Watteraer U, Ferstl FJ, Herbst EW, Schwarzkopf G, Hes FJ, Lips CJ, Lamiell JM, Masek O, Riegler P, Mueller B, Glavac D, Brauch H (1998) Prevalence, morphology and biology of renal cell carcinoma in von Hippel-Lindau disease compared to sporadic renal cell carcinoma. J Urol 160:1248–1254 10.1097/00005392-199810000-00011 [DOI] [PubMed] [Google Scholar]
- Neumann HPH, Brauch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehöher C, Zerres K, Januszewicz A, Eng C (2002) Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346:1459–1466 10.1056/NEJMoa020152 [DOI] [PubMed] [Google Scholar]
- Nickerson M, Warren M, Toro J, Matrosova V, Glenn G, Turner M, Duray P, Merino M, Choyke P, Pavlovich C, Sharma N, Walther M, Munroe D, Hill R, Maher E, Greenberg C, Lerman M, Linehan W, Zbar B, Schmidt L (2002) Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with Birt-Hogg-Dube syndrome. Cancer Cell 2:157–164 10.1016/S1535-6108(02)00104-6 [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavleitch N, Chau V, Kalein WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2:423–427 10.1038/35017054 [DOI] [PubMed] [Google Scholar]
- Sampson JR, Patel A, Mee AD (1995) Multi-focal renal cell carcinoma in sibs from a chromosome 9-linked (TSC1) tuberous sclerosis family. J Med Genet 32:848–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, et al (1997) Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16:68–73 [DOI] [PubMed] [Google Scholar]
- Tacchini L, Dansi P, Mattucci E, Desiderio MA (2001) Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 heptoma cells. Carcinogenesis 22:1363–1371 10.1093/carcin/22.9.1363 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kahoski R, Gross D, Nicol D, Teh BT (2002) Familial adult renal neoplasia. J Med Genet 39:1–5 10.1136/jmg.39.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson IPMT, Alam NA, Rowan AJ, Barclay E, Kelsell D, Leigh I, Gorman P, et al (2002) Germline mutations in the fumarate hydratase gene predispose to dominantly inherited uterine fibroids, skin leiomyomata and renal cell cancer. Nat Genet 30:406–410 10.1038/ng849 [DOI] [PubMed] [Google Scholar]