Abstract
Hypergonadotropic ovarian failure is a common cause of female infertility. It is a heterogeneous disorder that, in the most severe forms, is a result of ovarian dysgenesis (OD). Most OD cases are associated with major X-chromosome abnormalities, but the pathogenesis of this disorder is still largely undefined in patients with a normal karyotype. Animal models showed the important role in female reproduction played by the product of a gene located at Xp11.2 in humans (BMP15). BMP15 is an oocyte-specific growth/differentiation factor that stimulates folliculogenesis and granulosa cell (GC) growth. We report two sisters with a normal karyotype who are affected with hypergonadotropic ovarian failure due to OD. The familial presentation suggested a genetic origin, and candidate genes were screened for mutations. A heterozygous nonconservative substitution in the pro region of BMP15 (Y235C) was identified in both sisters but not in 210 control alleles. This mutation was inherited from the father. Mutant BMP15 appears to be processed abnormally, is associated with reduced GC growth, and antagonizes the stimulatory activity of wild-type protein on GC proliferation. In conclusion, the first natural mutation in human BMP15 is associated with familial OD, indicating that the action of BMP15 is required for the progression of human folliculogenesis. This condition represents an exceptional example of X-linked human disease exclusively affecting heterozygous females who inherited the genetic alteration from the unaffected father. BMP15 defects are involved in the pathogenesis of hypergonadotropic ovarian failure in humans.
Hypergonadotropic ovarian failure is a heterogeneous disorder that, in the most severe forms, is a result of ovarian dysgenesis (OD [MIM 233300]). OD accounts for about half of the cases of primary amenorrhea (Timmreck and Reindollar 2003). Most OD cases are associated with major X chromosome abnormalities. Accordingly, genetic studies have identified several loci at Xq and Xp11.2–p22.1 whose functions are relevant for ovarian development (Zinn et al. 1998; Simpson and Rajkovic 1999; Marozzi et al. 2000). The etiopathogenesis of this disorder is still largely undefined in patients with a normal karyotype. Severe gonadotropin receptor defects are, indeed, a rare cause of OD in 46,XX women (Aittomaki et al. 1995; Layman et al. 1998), suggesting the possible involvement of other candidate genes. Recently, animal models showed the important role in ovarian development and folliculogenesis played by the paracrine action of growth/differentiation factors (GDF9 and GDF9b or BMP15) that originate fromoocyte cells (Dube et al. 1998; Galloway et al. 2000; Yan et al. 2001; Hanrahan et al. 2004). BMP15 (MIM 300247), in particular, is the product of a gene located at Xp11.2 in humans (Dube et al. 1998). Bmp15 knockout female mice are subfertile and show reduced ovulation rates after gonadotropin treatment (Yan et al. 2001). Natural mutations in the Bmp15 gene in sheep, termed “fecundity X Inverdale and Hanna” (FecXI/H), have provided insight into the role of BMP15 in female reproduction (Galloway et al. 2000). Ewes with heterozygous mutations exhibited increased ovulation and lambing rates, whereas severe infertility with an early block in folliculogenesis was seen in ewes with homozygous mutations. In humans, BMP15 transcripts could be detected in gonads by RT-PCR. In situ hybridization localized the transcript to the oocyte of developing follicles (Aaltonen et al. 1999). BMP15 is a growth/differentiation factor, a member of the transforming growth factor-β (TGF-β) superfamily (Chang et al. 2002). Members of this superfamily control many aspects of development by binding and activating two types of transmembrane serine/threonine kinase receptors (Chang et al. 2002). Functional studies have shown that BMP15 stimulates GC growth and promotes the progression of folliculogenesis from the primary stage to the FSHdependent stage (Otsuka et al. 2000; Chang et al. 2002; Shimasaki et al. 2004).
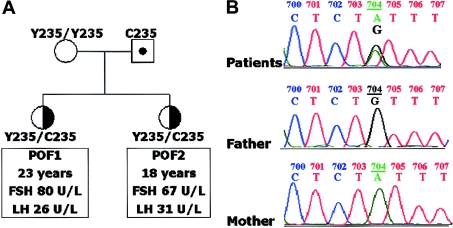
An Italian family with two female siblings affected with hypergonadotropic ovarian failure (fig. 1A) came to our attention recently. At 23 years old, the proband (VB) presented with primary amenorrhea and modest hirsutism (Ferriman-Gallwey score = 12 [Ferriman and Gallwey 1961]; normal values are <8). She was born at term after an uneventful pregnancy. Her physical and intellectual development were fairly normal until puberty. Owing to the lack of spontaneous menarche, the diagnosis of pubertal delay was given when she was 15 years old, but no treatment was initiated. At age 17 years, she underwent appendectomy, and laparoscopic investigation allowed the visualization of streak ovaries with a small terminal crest and hypogenesia of the uterus. She was put on estroprogestin therapy soon after surgery. Family history revealed that her younger sister (SB) was also affected with a similar menstrual defect, reporting a single episode of spotting at 13 years. At the time of this clinical evaluation, SB was 18 years old and had been receiving estroprogestins for 18 mo. Both patients had hypoplasic gonads at ultrasound (ovarian diameters <18 mm, with a homogeneous structure and without visible follicles) and a 46,XX karyotype. Their BMIs were 24 and 22 kg/m2, respectively, and all other endocrine functions were normal. Anti-ovary autoantibodies were negative on two occasions and there was no evidence of other specific autoimmune disease. Fertility and gonadal function were unaffected in both parents. The parents of VB and SB were not consanguineous, and clinical history of the family was negative for reproductive, endocrine, or mental disorders in the two previous generations.
Figure 1.
Pedigree and sequence analysis. A, Pedigree of the family. The elder sister (VB) and her younger sister (SB) were affected with idiopathic hypergonadotropic ovarian failure and presented with primary amenorrhea. After a 3-mo estrogen withdrawal, estradiol was low (<0.1 nmol/L) and gonadotropin values rose into the postmenopausal range, as shown (premenopausal female values: FSH = 1.0–8.0 U/L; LH = 0.5–9.0 U/L). The mother had regular menses and was postmenopausal at the time of the study (physiological menopause at 49 years), and the father had normal fertility and gonadal function (FSH = 5.3 U/L; LH = 2.5 U/L; testosterone = 15.6 nmol/L). B, BMP15 sequence analyses in VB, SB, and the parents. In the proband (VB), automated sequencing revealed a heterozygous A→G transition at base pair 704 of the BMP15 gene located at Xp11.2. The same heterozygous transition was seen in the younger sister, whereas the father was a hemizygous carrier of the same 704A→G transition and the mother was normal. The grandmother, two uncles, and two aunts of the paternal family had normal fertility (five, one, one, four, and three births, respectively). One of these aunts had a normal BMP15 sequence; therefore, the mutation should have arisen de novo in the hemizygous father. This transition generates a missense substitution (Y235C) located in the propeptide region of the BMP15 protein.
We decided to screen candidate genes for OD in this family. An institutional ethical committee approved the study protocol, and informed consent and blood samples for genetic investigations were obtained from all family members.
The phenotype of these patients was similar to that observed in patients with complete resistance to FSH action (Aittomaki et al. 1995). Accordingly, we had excluded mutations in the FSH-receptor coding sequence in the proband (see methods in appendix A [online only]). Because of evidence in animal models, investigations were focused on genes encoding for oocyte-specific growth/differentiation factors of the TGF-β superfamily (Chang et al. 2002; Shimasaki et al. 2004). A Japanese group (Takebayashi et al. 2000) reported the absence of BMP15 and GDF9 mutations in women with postpubertal onset of hypergonadotropic ovarian failure and secondary amenorrhoea or with polycystic ovaries. In the two sisters, the GDF9 coding sequence (GenBank accession number NM_005260) was completely normal, but we found a heterozygous A→G transition at base pair 704 of the BMP15 gene (GenBank accession number AF082349) in the constitutive DNA of VB (fig. 1B; see methods in appendix A [online only]). The same heterozygous transition was seen in SB, whereas the father was a hemizygous carrier of the same transition, and the wild-type BMP15 coding sequence was seen in the mother. This transition does not represent a polymorphic variant diffuse in the white population, as it was absent in a series of 210 alleles from 120 ethnically matched controls (90 women and 30 men). Thus, this heterozygous transition of the BMP15 gene leads to a Y235C mutation associated with prepubertal onset of hypergonadotropic ovarian failure characterized by primary amenorrhea and ovarian hypoplasia. These manifestations reflect the phenotype of sheep with homozygous mutations, rather than that of heterozygous ewes (Galloway et al. 2000; Hanrahan et al. 2004). The possibility that the affected sisters inherited additional alterations in other genes involved in ovarian function may explain such discrepancy. Nevertheless, mutations in GDF9 and FSHR genes were not detected, possibly suggesting that the different modes of inheritance in humans and sheep may be a result of species diversity or the peculiar nature and location of the human mutation.
The missense mutation lies in the second exon, in a highly conserved part of the BMP15 gene encoding the propeptide region (fig. 2). The pro region plays important biological roles, with a significant impact on secretion and action of TGF-β superfamily members (Chang et al. 2002). All TGF-βs are, indeed, synthesized as prepropeptide precursors (comprising the signal peptide, pro region, and bioactive mature domain) (Chang et al. 2002). The processing of TGF-β precursors is a critical step that modulates the availability of bioactive peptides at the target tissue level. Accordingly, TGF-βs are secreted partially as latent forms, and the pro region is cleaved by specific proteases in the intracellular or extracellular compartments as an important component of the posttranslational processing that leads to the release of the bioactive mature peptides (Gentry et al. 1988; Schultz-Cherry et al. 1994; Saharinen et al. 1999; Chang et al. 2002; Liao et al. 2003). Moreover, the pro region is involved in dimerization, and an essential role in this process was assigned to three Cys residues in the pro region of TGF-β1 (Brunner et al. 1989). The wild-type (WT) BMP15 lacks these characteristic cysteines. On the basis of all this data, the replacement of the highly conserved Tyr235 with a Cys was predicted to cause relevant modifications in the conformation of the BMP15 precursor, possibly leading to altered processing and impaired activation of latent forms or to abnormal dimerization.
Figure 2.
Alignment of the amino acid sequence of part of the BMP15 propeptide region from several mammalian species with its human homologue GDF9. The tyrosine affected by the mutation and the surrounding residues are highly conserved.
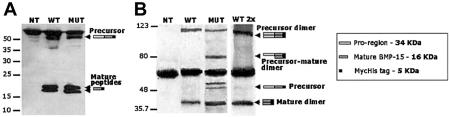
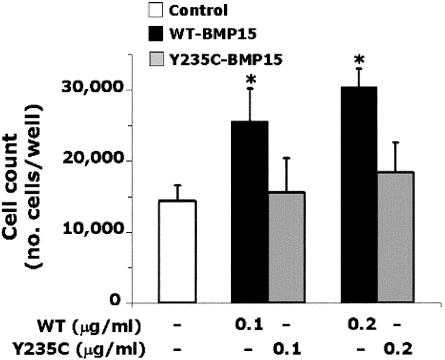
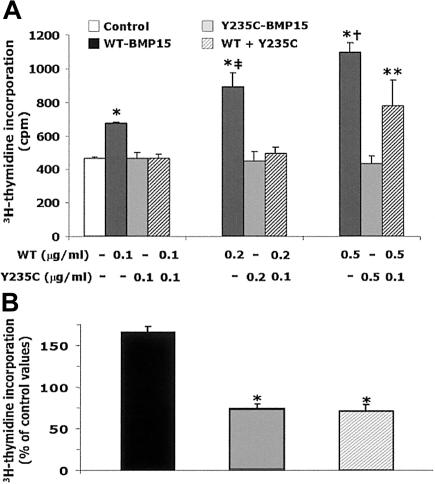
For functional studies, stable clones of human embryonic kidney 293T (HEK293T) cells expressing WT BMP15 or Y235C mutant BMP15 were obtained (see methods in appendix A [online only]). Western blotting qualitatively investigated immunoprecipitated BMP15 forms secreted by HEK293T cells. Under reducing conditions (fig. 3A), bands corresponding to mature and precursor BMP15-Myc-His chimeras were seen in the WT and mutant lanes, indicating that significant amounts of processed or unprocessed fusion proteins were secreted into the culture media in both cases. Under nonreducing conditions (fig. 3B), bands corresponding to mature and precursor dimers were seen in the WT and mutant lanes, but additional bands corresponding to precursor monomers or to stable precursor-mature dimers were visible only in the mutant lane, even when a double amount of WT protein was tested. These features are consistent with an altered processing of the Y235C mutant. The introduction of the Cys into the BMP15 pro region may confer to the mutant precursor the ability to form covalent heterodimers with mature peptides through the formation of abnormal disulfide bonds. The biological impact of the Y235C mutation is demonstrated with GC growth assays (Tapanainen et al. 1987) (appendix A [online only]). Human GCs were obtained by centrifugation of follicular fluids after the oocyte pick-up procedure in women undergoing in vitro fertilization. The women gave their informed consent. Cell count showed that GCs proliferated in the absence of recombinant BMP15 constructs (control cell count = 143% of basal). The proliferation rate of GCs was stimulated by the addition of WT (+79% or +113% of control values of 0.1 or 0.2 μg/ml, respectively), whereas no significant variation was seen in the presence of both doses of mutant (fig. 4). Accordingly, WT-BMP15 significantly stimulated 3H-thymidine incorporation in GCs, with a dose-response effect between 0.1 and 0.5 μg/ml (fig. 5A). In contrast, GC growth was not stimulated in the presence of increasing Y235C-BMP15 doses or when GCs were incubated with equal amounts (1:1) of WT and mutant proteins. A significant increase of 3H-thymidine incorporation in GCs was restored in the presence of WT-BMP15 concentrations 5-fold higher than those of Y235C-BMP15 (5:1). Despite heterogeneity among diverse GC preparations with variable basal growth rates, reciprocal differences between different BMP15 preparations were conserved in eight distinct experiments (fig. 5B).
Figure 3.
Western immunoblotting was performed under reducing and nonreducing conditions (with or without β-mercaptoetanol [β-ME]) by use of anti-His-HRP antibody (Roche) or anti-Myc antibody (Invitrogen), as appropriate. Immunofluorescent signals were detected with the use of the ECL system (Amersham). Western blots performed with anti-Myc antibody are shown. A, Analysis performed in the presence of β-ME. Bands corresponding to mature (duplet at ∼21–23 kDa) and precursor (at ∼50–55 kDa) BMP15-Myc-His chimeras were seen in the WT and mutant (MUT) lanes but were not seen in the control media from nontransfected (NT) cells. B, Analysis performed under nonreducing conditions allowing the visualization of dimeric products. Bands corresponding to mature (∼45 kDa) and precursor (>110 kDa) dimers were seen in both WT and MUT lanes. Additional bands corresponding to precursor monomers (∼50–55 kDa) or to stable precursor-mature dimers (∼80 kDa) were detected in the mutant Y235C-BMP15 but not in WT lanes, even when double the amount of protein was tested (WT 2x). The band at 65–70 kDa in both panels corresponds to a nonspecific cross-reacting protein present in all media.
Figure 4.
Granulosa cell-growth assay evaluated by direct cell count (see methods in appendix A [online only]). Results (mean ± SD) of a representative assay using different doses of BMP15-Myc-His fusion proteins. Tagged proteins were purified on nickel columns, and doses of purified proteins (0.1 or 0.2 μg/ml) were tested in triplicate wells. An asterisk (*) indicates P<.05 versus control and mutant preparations (two-tailed, unpaired Student’s t test).
Figure 5.
Granulosa cell-growth assay evaluated by 3H-thymidine incorporation (see methods in appendix A [online only]). A, Results (mean ± SD) of a representative assay using different doses of BMP15-Myc-His fusion proteins. Tagged proteins were obtained after affinity chromatography on nickel columns. Mutant BMP15 and WT-BMP15 were tested either separately or in combination (1:1 or 1:5 mixtures) in triplicate wells. An asterisk (*) indicates P<.01 versus control and mutant alone; a double dagger (‡) indicates P<.02 versus WT 0.1 μg/ml; a dagger (†) indicates P<.03 versus WT 0.2 μg/ml; and two asterisks (**) indicate P<.03 versus control and mutant alone (two-tailed, unpaired Student’s t test). B, Effects of recombinant human BMP15 tagged proteins (0.2 μg/ml) on GC growth, tested in eight experiments with the use of different GC preparations from eight women undergoing ovarian hyperstimulation. Owing to variable growth rates among these different GC preparations, results (mean ± SE; n=8) obtained with WT, mutant, or 1:1 mixtures are expressed as the percentage of 3H-thymidine incorporation in control wells. In WT-BMP15 wells, growth rate was 165% ± 6% (mean ± SE) of control wells (P<.001). In contrast, growth rates were reduced significantly, by >2-fold, when mutant BMP15 was tested separately or in the presence of equal amounts (1:1) of WT (74% ± 4% or 69% ± 6% of controls, respectively). An asterisk (*) indicates P<.001 versus WT-BMP15 (two-tailed, unpaired Student’s t test).
In summary, these experiments showed that WT-BMP15 stimulates human GC growth in a dose-dependent manner and that Y235C-BMP15 products lost stimulatory activity. When equal amounts of WT and mutant BMP15 preparations were coincubated with GCs, the abolition of WT stimulatory action was seen. Therefore, Y235C-BMP15 appears to generate a dominant negative effect on WT-BMP15 action. The formation of abnormal dimers producing a potent dominant negative effect by preventing the secretion of bioactive proteins was reported elsewhere in the case of the C400Y mutation of a BMP-like protein, the cartilage-derived morphogenetic protein-1 in an autosomal dominant form of chondrodysplasia (Thomas et al. 1997). Similarly, BMP15 mutations in sheep (Galloway et al. 2000) were reported to impair intracellular processing and the secretion of bioactive peptides (Liao et al. 2003). The dominant negative effect exerted by Y235C-BMP15 may be explained by means of two different molecular mechanisms: (1) impaired pro-protein processing and reduced production of bioactive peptides and (2) secretion of unprocessed monomer or dimeric mutant products with an antagonistic effect on GCs at the target level. The relevance of the latter mechanism was confirmed by GC proliferation assay through the abrogation of WT-BMP15 growth effect when WT protein was coincubated with equal amounts of mutant. Though specific BMP15 receptors are still not defined, binding to two distinct receptor types on GC membranes is expected for the generation of BMP15 signal (Chang et al. 2002; Moore et al. 2003). It is conceivable that Y235C-BMP15 unprocessed products (either monomer or abnormal dimer) may interact with one of the two receptor isoforms required for signal transduction in GCs, thus limiting receptor availability for WT-BMP15 action and creating a functional antagonism. Consistently, the antagonism exerted by mutant products can be overcome partially by the addition of 5-fold higher WT-BMP15 concentrations. A similar mechanism was described to modulate the activity of another member of the TGF-β superfamily, that is, the antagonism on activin function generated by inhibin binding to betaglycan (Lewis et al. 2000). Although evidence in animal models (Galloway et al. 2000; Hanrahan et al. 2004) suggests that additional genetic alterations may be required to generate the clinical phenotype of our patients, the present experimental findings indicate that Y235C-BMP15 could antagonize the activity of WT protein. The biological relevance in vivo of the partial processing defect may also develop from the peculiar type of BMP15 paracrine action in the restricted follicle compartment.
In conclusion, the diminished GC proliferation observed in vitro in the presence of Y235C-BMP15 can explain the in vivo phenotype of primary amenorrhoea and hypoplasic ovaries in the two 46,XX sisters with the BMP15 mutation. The hemizygous father was apparently unaffected. Extragonadal tissues known to express BMP15 include the pituitary (Otsuka and Shimasaki 2002), but normal gonadotropin secretion in the father argues against an essential role for BMP15 at this level. Our data indicate that mutations in BMP15 gene can be associated with hypergonadotropic ovarian failure in humans and that BMP15 is one of the factors whose function is relevant for OD in Turner syndrome. Owing to the essential role, limited to the female sex, of the growth/differentiation pathway affected by BMP15 mutation (Dube et al. 1998; Aaltonen et al. 1999; Galloway et al. 2000; Otsuka et al. 2000; Yan et al. 2001; Chang et al. 2002; Hanrahan et al. 2004; Shimasaki et al. 2004), this condition represents an exceptional example of X-linked human disease exclusively affecting heterozygous females who inherited the genetic alteration from their unaffected fathers.
Acknowledgments
We are grateful to the family members for their participation in the study. We are indebted to Drs. Sara Pariani, PaolaSerafini, and Veronica Cozzi, for the supply of follicular materials from women undergoing stimulation protocols for in vitro fertilization at the Fertility Unit of the Division of Gynecology and Obstetrics of San Paolo Hospital, Milan. We wish to thank Professor Giovanni Faglia for continuous support and critical reading of the manuscript. This work was supported in part by grants from the Italian Ministry of Instruction, University, and Research (MIUR) (2002068221-001 to L.P.) and from Funds of Istituto Auxologico Italiano IRCCS (project MOLINF) to L.P.
Appendix A: Supplemental Material
Sequence Analysis
Genomic DNA was extracted from circulating leukocytes and from FSHR, BMP15, and GDF9 coding sequences, and intron-exon boundaries were analyzed with the use of intronic primer pairs. The primers for FSHR analysis have been reported elsewhere (Aittomaki et al. 1995). The primers used for BMP15 and GDF9 analyses are as follows. BMP15: 1F, 5′-AGTGACGTCCCTTGGGCTTG-3′, and 1R, 5′-CAAAGCCTGACAGTAAACCC-3′; 2AF, 5′-CTATCAGTCTATATCAAGACAG-3′, and 2AR, 5′-GCTCAAGACCACCACTATCT-3′; and 2BF, 5′-GGTTCTGGAATAACAAGGGAC-3′, and 2BR, 5′-AACCTACAGATTGGTACAGGAT-3′. GDF9: 1F, 5′-AGGAAGAGGACTGGCATGG-3′, and 1R, 5′-GTGTCTATCATCTTCCCTCC-3′; 2AF, 5′-TTGACTTGACTGCCTGTTGTG-3′, and 2AR, 5′-AGATCTCCCATCCTCAGCAG-3′; and 2BF, 5′-TGAATGACACAAGTGCTCAGG-3′, and 2BR, 5′-GCACACAGTAGTTACTTTGCC-3′. Amplified fragments were sequenced on the ABI PRISM 310 sequence analyser (Applied Biosystems).
Plasmid Construction and Production of BMP15-Myc-His Chimeras
A full-length human BMP15 cDNA was amplified by RT-PCR from human testes total RNA, with the use of specific primers containing the cleavage sites for the NheI and XhoI restriction enzymes. An amplified fragment was inserted into the eukaryotic expression vectorpCDNA4Myc-His (Invitrogen) containing the zeocin resistance gene, to generate a fusion protein BMP15-Myc-His. The 704A→G mutation was introduced with the use of the Quick Change Site-directed Mutagenesis Kit (Stratagene).
HEK293T cells were transfected stably with WT or mutant BMP15 cDNAs, by the calcium-phosphate precipitation method. HEK293T cells were shown to lack endogenous expression of BMP15 or GDF9 (Liao et al. 2003). Stable clones expressing WT or mutant BMP15 were selected with the use of 600 μg/ml of zeocin. Selected clones were maintained in a medium containing 10% foetal bovine serum (FBS) and zeocin (400 μg/ml). At 90% confluence, cells were switched to a medium containing 1% FBS, and the conditioned mediumwas harvested after 3 d. A medium of nontransfected HEK293T cells was used as a control. Tagged proteins were extracted initially from media, with the use of either nickel resin columns (Invitrogen) for purification of 6His-tagged products or with the use of anti-Myc antibody preadsorbed to protein G sepharose (Amersham). Results of western blot analysis were quite similar for both protein preparations. Owing to their higher capacity, nickel columns were chosen for the preparation of larger amounts of partially purified proteins, for all the studies that are illustrated here. The amount of recovered protein was evaluated by colorimetric assay (Pierce). The total amount of purified proteins recovered from differently conditioned media ranged from 499–848 μg.
GC Growth Assay
The potency of WT and mutant BMP15 in stimulating GC proliferation was investigated by direct cell count (Z2 Coulter Counter, Beckman-Coulter) or by 3H-thymidine incorporation, following the protocol described by Tapanainen et al. (1987). To obtain a sufficient number of cells, we collected GCs after in vivo hyperstimulation. Isolated GCs were maintained in medium-199 (GIBCO) supplemented with glutamine, 10% FBS, and 50 μg/ml of gentamicin. For growth assays, GCs were seeded in 24-well dishes at the density of 10,000/well for cell count and 40,000/well for 3H-thymidine incorporation. After 24 h, purified recombinant BMP15 preparations were added to the culture medium in triplicate wells, in the presence or absence of methyl–3H-thymidine (0.5 μCi/well). WT and mutant BMP15 were tested either separately or in combination. This was done by adding mixtures containing equal amounts of both proteins (1:1) or mixtures containing 5-fold higher amounts of WT (1:5) to the same well. Preparations of the medium of nontransfected HEK293T cells were used as a control. GCs were harvested 24 h after the addition of stimuli. For cell count, cells were collected in 20 ml of isotonic solution and counted in triplicates. For 3H-thymidine incorporation, GCs were washed in PBS and treated with 10% ice cold trichloroacetic acid for 30 min at 4°C. The cell pellets were resuspended in 1M NaOH and radioactivity was measured in a β-counter after sample buffering. Assay data were analyzed by Student’s t test, as appropriate. Differences were considered statistically significant if P<.05.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human BMP15 genomic sequence [accession number AF082349] and human GDF9 mRNA sequence [accession number NM_005260])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R, Hovata O, Dale L, Ritvos O (1999) Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab 84:2744–2750 10.1210/jc.84.8.2744 [DOI] [PubMed] [Google Scholar]
- Aittomaki K, Lucena JLD, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de la Chapelle A (1995) Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82:959–968 [DOI] [PubMed] [Google Scholar]
- Brunner AM, Marquardt H, Malacko AR, Lioubin MN, Purchio AF (1989) Site directed mutagenesis of cysteine residues in the pro region of the transforming growth factor β1 precursor. J Biol Chem 264:13660–13664 [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23:787–823 10.1210/er.2002-0003 [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM (1998) The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 12:1809–1817 10.1210/me.12.12.1809 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF (2004) The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101 10.1210/er.2003-0007 [DOI] [PubMed] [Google Scholar]
- Ferriman D, Gallwey JD (1961) Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 21:1440–1447 [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O (2000) Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 10.1038/77033 [DOI] [PubMed] [Google Scholar]
- Gentry LE, Lioubin MN, Purchio AF, Marquardt H (1988) Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor β to the mature polypeptide. Mol Cell Biol 8:4162–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM (2004) Mutations in genes for oocyte derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 70:900–909 [DOI] [PubMed] [Google Scholar]
- Layman LC, Amde S, Cohen DP, Jin M, Xie J (1998) The Finnish follicle-stimulating hormone receptor gene mutation is rare in North American women with 46,XX ovarian failure. Fertil Steril 69:300–302 10.1016/S0015-0282(97)00480-9 [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W (2000) Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411–414 10.1038/35006129 [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Otsuka F, Shimasaki S (2003) Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9: implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J Biol Chem 278:3713–3719 10.1074/jbc.M210598200 [DOI] [PubMed] [Google Scholar]
- Marozzi A, Manfredini E, Tibiletti MG, Tibiletti MG, Furlan D, Villa N, Vegetti W, Crosignani PG, Ginelli E, Meneveri R, Dalpra L (2000) Molecular definition of Xq common-deleted region in patients affected by premature ovarian failure. Hum Genet 107:304–311 10.1007/s004390000364 [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S (2003) Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 278:304–310 10.1074/jbc.M207362200 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S (2002) A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology 143:4938–4941 10.1210/en.2002-220929 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S (2000) Bone morphogenetic protein-15: identification of target cells and biological functions. J Biol Chem 275:39523–39528 10.1074/jbc.M007428200 [DOI] [PubMed] [Google Scholar]
- Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J (1999) Latent transforming growth factor-β binding proteins (LTBPs)-structural extracellular matrix proteins for targeting TGF-β action. Cytokine Growth Factor Rev 10:99–117 10.1016/S1359-6101(99)00010-6 [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Lawler J, Murphy-Ullrich JE (1994) The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J Biol Chem 269:26783–26788 [PubMed] [Google Scholar]
- Simpson JL, Rajkovic A (1999) Ovarian differentiation and gonadal failure. Am J Med Genet 89:186–200 [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Takakura K, Wang HQ, Kimura F, Kasahara K, Noda Y (2000) Mutation analysis of the growth differentiation factor-9 and -9b genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril 74:976–979 10.1016/S0015-0282(00)01539-9 [DOI] [PubMed] [Google Scholar]
- Tapanainen J, Leinonen PJ, Tapanainen P, Yamamoto M, Jaffe RB (1987) Regulation of human granulosa-luteal cell progesterone production and proliferation by gonadotropins and growth factors. Fertil Steril 48:576–580 [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP (1997) Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet 17:58–64 [DOI] [PubMed] [Google Scholar]
- Timmreck LS, Reindollar RH (2003) Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am 30:287–302 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866 10.1210/me.15.6.854 [DOI] [PubMed] [Google Scholar]
- Zinn AR, Tonk VS, Chen Z, Flejter WL, Gardner HA, Guerra R, Kushner H, Schwartz S, Sybert VP, Van Dyke DL, Ross JL (1998) Evidence for a Turner syndrome locus or loci at Xp11.2-p22.1. Am J Hum Genet 63:1757–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]