Abstract
Seckel syndrome (SCKL) is a rare, genetically heterogeneous disorder, with dysmorphic facial appearance, growth retardation, microcephaly, mental retardation, variable chromosomal instability, and hematological disorders. To date, three loci have been linked to this syndrome, and recently, the gene encoding ataxia-telangiectasia and Rad3-related protein (ATR) was identified as the gene mutated at the SCKL1 locus. The ATR mutation affects splicing efficiency, resulting in low levels of ATR in affected individuals. Elsewhere, we reported increased instability at common chromosomal fragile sites in cells lacking the replication checkpoint gene ATR. Here, we tested whether cells from patients carrying the SCKL1 mutation would show increased chromosome breakage following replication stress. We found that, compared with controls, there is greater chromosomal instability, particularly at fragile sites, in SCKL1-affected patient cells after treatment with aphidicolin, an inhibitor of DNA polymerase α and other polymerases. The difference in chromosomal instability between control and patient cells increases at higher levels of aphidicolin treatment, suggesting that the low level of ATR present in these patients is not sufficient to respond appropriately to replication stress. This is the first human genetic syndrome associated with increased chromosome instability at fragile sites following replication stress, and these findings may be related to the phenotypic findings in patients with SCKL1.
Seckel syndrome (SCKL [MIM #210600]) is a rare, genetically heterogeneous autosomal recessive disorder of proportionate dwarfism, mental retardation, and characteristic faces marked by microcephaly, craniosynostosis, receding forehead, narrow face, large beaked nose, and micrognathia, giving the face an asymmetric “bird-like” appearance. Since SCKL was first described in 1960 (Seckel 1960), >70 cases have been reported, but there is considerable heterogeneity in their clinical characteristics. This disorder shares many clinical characteristics with Nijmegen breakage syndrome (MIM #251260) and LIG4 syndrome (MIM #606593), both of which are syndromes involving DNA damage-response genes. Some patients exhibit café-au-lait spots (Woods et al. 1995; Børglum et al. 2001; Kilinç et al. 2003) and immunological and hematological disorders, including frequent infections, anemia (Lilleyman 1984), pancytopenia; one patient received a diagnosis of acute myeloid leukemia (Hayani et al. 1994).
There have been mixed reports of chromosome instability in cells from patients with SCKL. Nine patients have been reported to have increased chromosomal instability after treatment with mitomycin C (MMC) (Butler et al. 1987; Syrrou et al. 1995; Woods et al. 1995; Bobabilla-Morales et al. 2003), and two patients with spontaneous chromosomal instability have been reported (Butler et al. 1987; Woods et al. 1995). However, cells from two patients with unknown mutations have been reported to be resistant to MMC (Abou-Zahr et al. 1999). Increased sister-chromatid exchange (SCE) has also been observed (Cervenka et al. 1979; Syrrou et al. 1995).
It is not surprising, given the varied clinical findings, that several loci have been linked to SCKL. The first locus, SCKL1, was mapped by Goodship et al. (2000) to 3q22.1-q24 in two consanguineous Pakistani families. SCKL2 was mapped by Børglum et al. (2001) to 18p11.31-q11.2 in a consanguineous Iraqi family, and SCKL3 was mapped, by linkage analysis by Kilinç et al. (2003), to a 1.18-cM region on 14q in five Turkish families. Kilinç et al. (2003) also noted that the mutation in four other Turkish families did not show linkage to any of the three Seckel loci, indicating that additional genetic heterogeneity remains.
Recently, O’Driscoll et al. (2003) identified ATR (ataxia-telangiectasia and Rad3-related protein) as the gene mutated at the SCKL1 locus. The mutation is a silent 2101A→G transition in ATR exon 9 that results in increased aberrant splicing, either skipping of exon 9 or use of cryptic splice donors within exon 9, both of which introduce a frameshift and stop codon in exon 10. Patients homozygous for the mutation have a low level of correctly spliced ATR, resulting in low levels of ATR protein, as monitored by western blot (O’Driscoll et al. 2003).
ATR is a member of the PI3K family, is closely related to ATM, and functions in cell-cycle checkpoint and DNA-repair pathways. It is a key member of the intra-S and G2/M checkpoints and responds primarily to replication stress, such as that caused by hydroxyurea, aphidicolin, and hypoxia (Cliby et al. 1998; Cortez et al. 2001; Nghiem et al. 2001; Hammond et al. 2002). When activated, ATR stabilizes stalled replication forks (Lopes et al. 2001; Tercero and Diffley 2001), inhibits late origin firing (Tercero and Diffley 2001), and blocks progression from G2 into mitosis (Nghiem et al. 2001).
We recently determined that ATR is critical for the normal maintenance of stability at common fragile sites (Casper et al. 2002). Common fragile sites are specific loci that preferentially exhibit gaps and breaks on metaphase chromosomes under conditions that partially inhibit DNA replication (Glover et al. 1984). Although they are normally stable in cultured human cells, under replicative stress, fragile sites are “hot spots” for SCEs, translocations, and deletions (Glover and Stein 1987, 1988), as well as integration of foreign DNA (Popescu and DiPaolo 1989; Rassool et al. 1991; Smith et al. 1992; Mishmar et al. 1998), and they trigger some gene amplification events via a breakage-fusion-bridge cycle (Kuo et al. 1994; Coquelle et al. 1997; Hellman et al. 2002). Numerous studies have shown that fragile sites are unstable in tumors (reviewed in Huebner and Croce [2001]). Although the mechanism of instability at fragile sites is not yet fully understood, it is now known that the ATR-checkpoint pathway plays a critical role. We previously found that ATR deficiency results in a 5–20-fold increase in chromosomal gaps and breaks at fragile sites after aphidicolin treatment, as well as in the appearance of fragile site breaks even without the addition of replication inhibitors (Casper et al. 2002). Therefore, we hypothesized that cells from SCKL1-affected patients with a hypomorphic mutation in ATR would have increased chromosome instability, especially at fragile sites, following replication stress.
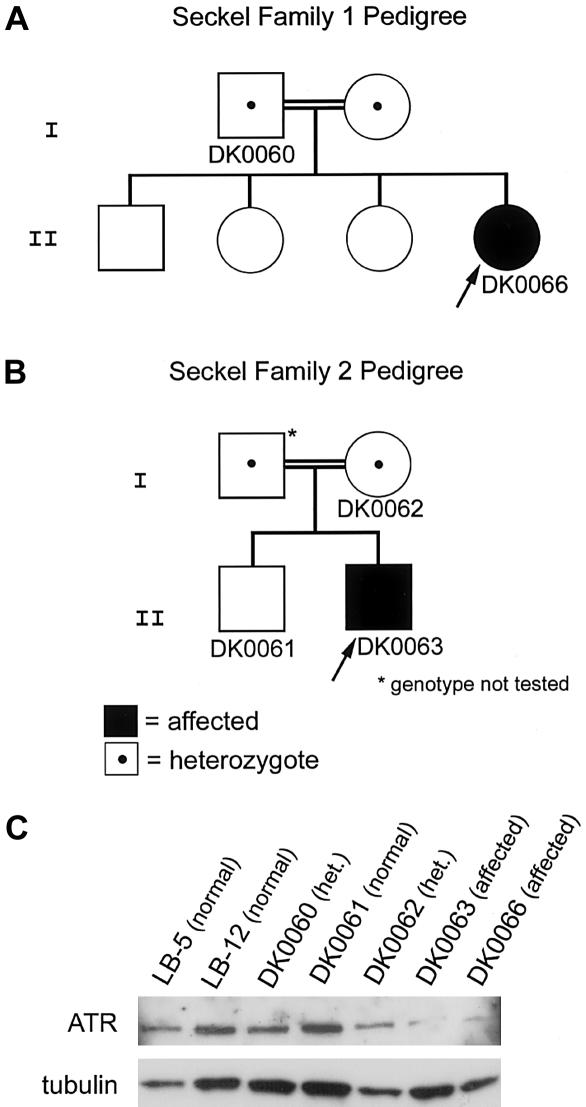
To test this hypothesis, we obtained Epstein-Barr virus (EBV)–transformed lymphoblast cell lines DK0060, DK0061, DK0062, DK0063, and DK0066 from two families with SCKL1 from J. Goodship (Newcastle University, Newcastle-upon-Tyne, United Kingdom) (Goodship et al. 2000; O’Driscoll et al. 2003) and LB-5 and LB-12, two EBV-transformed lymphoblast lines established from normal individuals. O’Driscoll et al. (2003) elsewhere reported a silent mutation in ATR in these two families with SCKL1, which resulted in aberrant ATR transcripts and a reduced ATR protein level in fibroblasts from one affected individual. We harvested cellular protein from all the lymphoblastoid cell lines for western blot analysis of ATR. ATR was detected with a rabbit polyclonal antibody generated against amino acids 1–20 of ATR, and the membrane was then stripped and reprobed with anti-tubulin. As expected, we found that cell lines DK0063 and DK0066 from affected patients have reduced ATR protein levels, compared with their heterozygous parent lines DK0060 and DK0062 and with normal control lines DK0061, LB-5, and LB-12 (fig. 1A–1C).
Figure 1.
Pedigrees of family with SCKL and western blot showing reduced ATR protein levels in affected patients. A and B, Pedigrees of the two Pakistani families with SCKL (SCKL1) included in this study. Identification numbers of the lymphoblast cell lines derived from these family members are noted below their respective symbols. C, Western blot of lymphoblast cell lines from these family members and two unrelated control lymphoblast lines (LB-5 and LB-12) probed with α-ATR. The blot was stripped and reprobed with α-tubulin to indicate relative protein-loading levels.
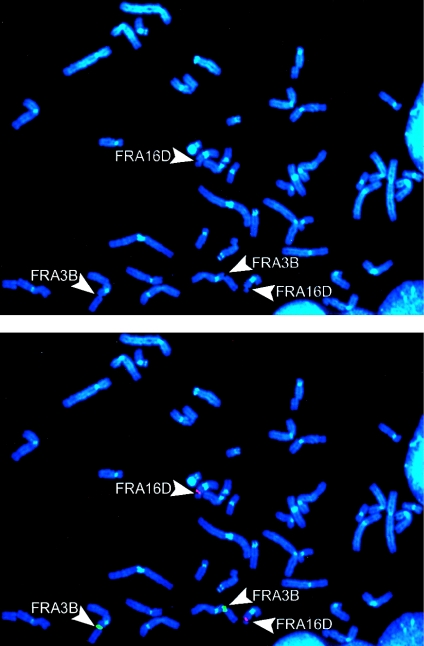
We next treated these lymphoblast lines with 0.4 μM aphidicolin for 48 h and harvested them for chromosome preparations, under standard conditions. Metaphase spreads were examined for total chromosomal gaps and breaks, and FRA3B and FRA16D were detected by FISH analysis by use of probes within these fragile sites (YAC 850A6 and BAC264L1 [RP-11], respectively). Probe labeling, hybridization, and immunologic detection were performed according to standard protocols (Wilke et al. 1996). Biotin-labeled probes were detected with avidin-FITC followed by anti–avidin-FITC; digoxigenin-labeled probes were detected with rhodamine-conjugated antibody followed by Texas Red anti-goat. Chromosomes were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (fig. 2). For all analyses, at least 50 cells from each of two replicates were scored for each data point, and a one-tailed t test was employed for comparisons of average overall gaps and breaks and for comparisons of the frequency of gaps and breaks at specific fragile sites.
Figure 2.
Metaphase showing an example of FISH for identification of fragile sites. This representative metaphase is from lymphoblast cell line DK0061, after treatment with 0.4 μM aphidicolin. FRA3B is indicated by hybridization with YAC probe 850A6 (green); both homologs are broken. FRA16D is indicated by hybridization with BAC probe 264L1 (red); both homologs are broken.
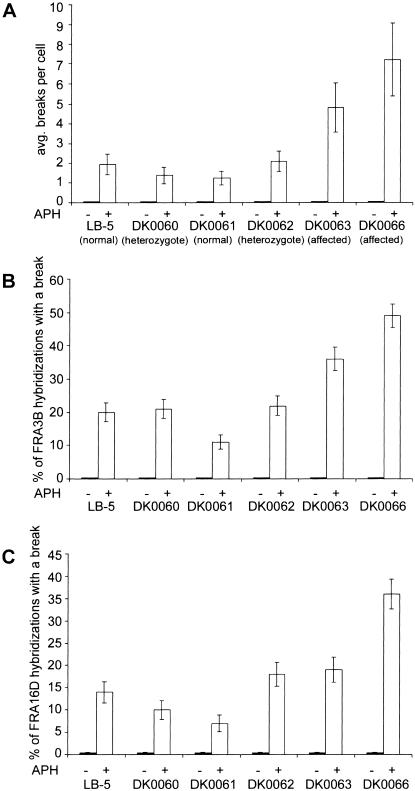
We found that, after aphidicolin treatment, the average total gaps and breaks per cell was increased 2.5–7-fold in SCKL1-affected patient lines, as compared with parent and control lines (fig. 3A). We did not observe any significant increase in spontaneous chromosomal instability in untreated cells. It is likely that the observed increase in chromosomal breaks is directly attributable to instability at fragile sites, since the majority of gaps and breaks observed after aphidicolin treatment occur at common fragile sites (Glover et al. 1984). To test this directly, we examined gaps and breaks at fragile sites FRA3B and FRA16D, two of the most frequently expressed common fragile sites. We found that FRA3B showed gaps and breaks 2–5-fold more often in SCKL1-affected patient lines than in parents and controls (fig. 3B). FRA16D is broken 2–5-fold more often in patient line DK0066, whereas FRA16D is not broken significantly more often in patient line DK0063 than in parent line DK0062 (fig. 3C). After treatment with 0.4 μM aphidicolin, we found that 22%–44% of all breaks occurred at FRA3B and FRA16D, which is consistent with earlier reports that the great majority of aphidicolin-induced gaps and breaks are at <20 fragile sites (Glover et al. 1984). These data suggest that breakage specifically at fragile sites is the cause of increased chromosomal instability in these SCKL1-affected cells after aphidicolin treatment.
Figure 3.
Cells from patients with SCKL1 have increased fragile-site expression. A, Average total chromosome gaps and breaks. n=50 metaphases from each of two replicates for each condition. Fragile-site induction was achieved by addition of 0.4 μM aphidicolin 48 h before harvest. Error bars indicate the 95% CI. B and C, Frequency of FRA3B (B) and FRA16D (C) expression in cell lines from patients with SCKL1 and control individual; n⩾100 hybridizations from each of two replicates for each condition. Fragile-site induction was achieved by addition of 0.4 μM aphidicolin 48 h before harvest. Error bars indicate the SE.
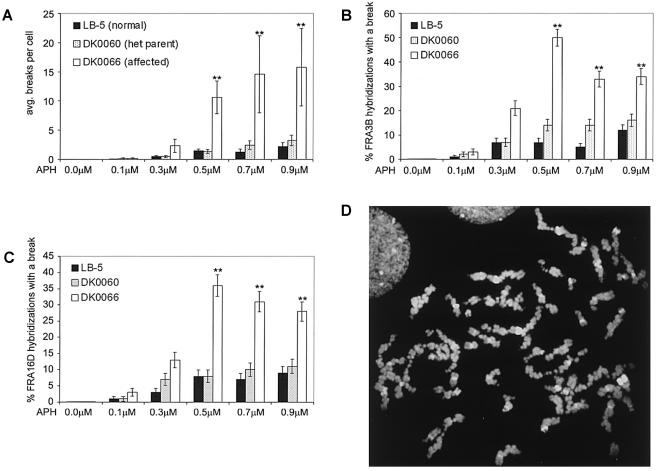
Since the ATR mutation in these SCKL1-affected patient lines is hypomorphic, we suspected that challenging these cells with higher doses of aphidicolin would reveal a threshold level of replication stress that these cells can accommodate, beyond which there is not enough functional ATR protein to respond appropriately. To test this hypothesis, we treated cell lines LB-5, DK0060, and DK0066 with increasing levels of aphidicolin—0.1–0.9 μM—for 48 h, and we examined metaphases from these cells in the same manner as described above. Following aphidicolin treatment at all concentrations, the average total breaks per cell was greater in SCKL1-affected cells than in controls (fig. 4A), and we found that the significance of this difference in average total breaks per cell increases at higher aphidicolin concentrations (table 1). The difference in gaps and breaks at both FRA3B and FRA16D between patients and controls is also more significant at higher aphidicolin concentrations (fig. 4B and 4C and table 1). We found that 10%–47% of all gaps and breaks occurred at just these two fragile sites. In the patient cell line DK0066 with 0.5 μM, 0.7 μM, and 0.9 μM aphidicolin treatment, we observed that 16%–38% of metaphases analyzed had a “shattered” appearance, with extreme chromosomal damage (as shown in fig. 4D). We did not observe any metaphases of this type in the normal LB-5 line or in the parental cell line DK0060. We did not see any “shattered” metaphases in the previous experiment after treatment with just 0.4 μM aphidicolin.
Figure 4.
The difference in fragile site breaks between patient and control cells increases at higher levels of aphidicolin treatment. Cells were treated with 0.1, 0.3, 0.5, 0.7, or 0.9 μM aphidicolin 48 h before harvest. Of DK0066 metaphases analyzed at high aphidicolin concentrations, 16%–38% had a “shattered” appearance and were not included in these calculations; thus, the figures reported are underestimates (indicated by a double asterisk [**]). A, Average total chromosome gaps and breaks. n=25 metaphases from each of two replicates for each condition. Error bars indicate the 95% CI. B and C, Frequency of FRA3B (B) and FRA16D (C) expression in cell lines from a patient with SCKL and a control individual; n⩾50 hybridizations from each of two replicates for each condition. Error bars indicate the SE. D, Example of a “shattered” metaphase in the affected cell line DK0066 after treatment with 0.5 μM aphidicolin for 48 h before harvest.
Table 1.
P Values of the Difference in Breaks after Aphidicolin Treatment
|
P Values after Aphidicolin Treatment with |
|||||
| Breaks and LymphoblastCell Linea | .1 μM | .3 μM | .5 μM | .7 μM | .9 μM |
| Total: | |||||
| LB-5 vs. DK0060 | .09799 | .47319 | .34485 | .00630 | .03574 |
| LB-5 vs. DK0066 | .02672 | .00136 | 2.86×10-8 b | 4.37×10-5 b | 1.14×10-4 b |
| DK0060 vs. DK0066 | .21772 | .00124 | 2.32×10-8 b | 1.34×10-4 b | 2.91×10-4 b |
| At FRA3B: | |||||
| LB-5 vs. DK0060 | 1.0 | 1.0 | .1652 | .0513 | .5416 |
| LB-5 vs. DK0066 | .6212 | .0072 | 1.00×10-10 | 6.66×10-7 b | 1.67×10-4 b |
| DK0060 vs. DK0066 | .9999 | .0072 | 6.20×10-8 | .0045b | .0030b |
| At FRA16D: | |||||
| LB-5 vs. DK0060 | 1.0 | .3311 | 1.0 | .6133 | .8143 |
| LB-5 vs. DK0066 | .6212 | .0165 | 2.17×10-6 | 5.09×10-5 b | .0017b |
| DK0060 vs. DK0066 | .6212 | .1676 | 2.17×10-6 | 7.70×10-4 b | .0071b |
LB-5=normal; DK0060=heterozygote; DK0066=affected.
Of DK0066 metaphases analyzed, 16%–38% had a “shattered” appearance and were not included in these calculations; thus, the significance reported is an underestimate.
In summary, we have found that SCKL1-affected patient cells with hypomorphic mutations in ATR have increased instability at fragile sites after aphidicolin treatment and that the significance of the difference in breaks between patient and control cells increases at higher levels of aphidicolin treatment, suggesting a threshold of replication stress for SCKL1 cells. Given the known cell-cycle checkpoint function of ATR (Abraham 2001), this increase in instability is probably due to improper progression into mitosis after failure to delay at the S-phase or G2/M checkpoints in response to replication stalling.
SCKL1 is the first human disease to be associated with mutations in ATR and with increased chromosome breakage at fragile sites, and these results affirm our earlier work that showed that ATR deficiency results in increased fragile-site instability (Casper et al. 2002). Furthermore, these results support earlier work indicating that the silent mutation in ATR in these families is causative for the SCKL1 phenotype (O’Driscoll et al. 2003). The malformations seen in these affected individuals may result from inappropriate checkpoint responses and chromosome breakage, leading to cell death at times of replicative stress during development. Since cancer or leukemia has not been reported in the two affected patients from these families, the ATR hypomorphic mutation and increased fragile site instability may not contribute to tumor initiation. However, both affected individuals are young (aged <15 years) (Goodship et al. 2000), and continued observation of the clinical phenotype of these patients offers the unique opportunity to understand the in vivo consequences of such instability on tumor initiation or progression. Since cells from these patients manage low levels of replication stress nearly as well as do normal cells but respond inappropriately at higher levels of stress, it is possible that patients with this mutation would be more likely to manifest clinical phenotypes in response to conditions known to challenge the ATR checkpoint pathway or induce fragile sites, such as folic acid deficiency. Folic acid deficiency, which is common worldwide, is known to induce breaks at common fragile sites (Glover et al. 1984), and has been associated with anemia, elevated serum homocysteine (which is associated with coronary artery disease and stroke), neural tube defects during pregnancy, and cancer development (Blount et al. 1997; Koury et al. 1997; Verhaar et al. 2002; Geisel 2003). In addition to the insight offered by clinical phenotypes, we believe that the hypomorphic ATR mutation itself will prove to be a useful tool for studying the consequences of ATR deficiency and impaired cellular responses to replication stalling. The level of ATR protein present after this mutation is high enough to prevent the cell death phenotype of ATR-null cells yet is low enough to result in deficient cell-cycle checkpoint responses in response to replication stress. Finally, it will be of interest to investigate fragile-site instability in cells from SCKL-affected patients that are linked to mutations on 18p11.31-q11.2 (SCKL2) and 14q (SCKL3), as well as from other patients with genetically undefined SCKL, to determine whether these complementation groups have mutations in genes related to the ATR checkpoint pathway.
Acknowledgments
We thank J. Goodship, for lymphoblast cell lines from the two Pakistani families with SCKL, and P. Nghiem, for the ATR antibody. We also thank M. O’Driscoll, J. Moran, and N. Howlett for helpful discussions. This work was supported by National Institutes of Health grant CA43222 (to T.W.G.) and a National Science Foundation Predoctoral Fellowship (to A.M.C.).
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SCKL, Nijmegen breakage syndrome, and LIG4 syndrome)
References
- Abou-Zahr F, Bejjani B, Kruyt FAE, Kurg R, Bacino C, Shapira SK, Youssoufian H (1999) Normal expression of the Fanconi anemia proteins FAA an FAC and sensitivity to mitomycin C in two patients with Seckel syndrome. Am J Med Genet 83:388–391 [DOI] [PubMed] [Google Scholar]
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15:2177–2196 10.1101/gad.914401 [DOI] [PubMed] [Google Scholar]
- Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN (1997) Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA 94:3290–3295 10.1073/pnas.94.7.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobabilla-Morales L, Corona-Rivera A, Corona-Rivera JR, Buenrostro C, García-Cobián TA, Corona-Rivera E, Cantú-Garza JM, García-Cruz D (2003) Chromosome instability induced in vitro with mitomycin C in five Seckel syndrome patients. Am J Med Genet 123A:148–152 14598338 [DOI] [PubMed] [Google Scholar]
- Børglum AD, Balslev T, Haagerup A, Birkebæk N, Binderup H, Kruse TA, Hertz JM (2001) A new locus for Seckel syndrome on chromosome 18p11.31-q11.2. Eur J Hum Genet 9:753–757 10.1038/sj.ejhg.5200701 [DOI] [PubMed] [Google Scholar]
- Butler MG, Hall BD, Maclean RN, Lozzio CB (1987) Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am J Med Genet 27:645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper AM, Nghiem P, Arlt MF, Glover TW (2002) ATR regulates fragile site stability. Cell 111:779–789 10.1016/S0092-8674(02)01113-3 [DOI] [PubMed] [Google Scholar]
- Cervenka J, Tsuchiya H, Ishiki T, Suzuki M, Mori H (1979) Seckel’s dwarfism: analysis of chromosome breakage and sister chromatid exchanges. Am J Dis Child 133:555–556 [DOI] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH (1998) Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J 17:159–169 10.1093/emboj/17.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M (1997) Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplicons. Cell 89:215–225 10.1016/S0092-8674(00)80201-9 [DOI] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ (2001) ATR and ATRIP: partners in checkpoint signaling. Science 294:1713–1716 10.1126/science.1065521 [DOI] [PubMed] [Google Scholar]
- Geisel J (2003) Folic acid and neural tube defects in pregnancy: a review. J Perinat Neonatal Nurs 17:268–279 [DOI] [PubMed] [Google Scholar]
- Glover TW, Berger C, Coyle J, Echo B (1984) DNA polymerase α inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet 67:136–142 [DOI] [PubMed] [Google Scholar]
- Glover TW, Stein CK (1987) Induction of sister chromatid exchanges at common fragile sites. Am J Hum Genet 41:882–890 [PMC free article] [PubMed] [Google Scholar]
- ——— (1988) Chromosome breakage and recombination at fragile sites. Am J Hum Genet 43:265–273 [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Gill H, Carter J, Jackson A, Splitt M, Wright M (2000) Autozygosity mapping of a Seckel syndrome locus to chromosome 3q22.1-q24. Am J Hum Genet 67:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ (2002) Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol 22:1834–1843 10.1128/MCB.22.6.1834-1843.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayani A, Suarez CR, Molnar Z, LeBeau M, Godwin J (1994) Acute myeloid leukaemia in a patient with Seckel syndrome. J Med Genet 31:148–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A, Zlotorynski E, Scherer SW, Cheung J, Vincent JB, Smith DI, Trakhtenbrot L, Kerem B (2002) A role for common fragile site induction in amplification of human oncogenes. Cancer Cell 1:89–97 10.1016/S1535-6108(02)00017-X [DOI] [PubMed] [Google Scholar]
- Huebner K, Croce CM (2001) FRA3B and other common fragile sites: the weakest links. Nat Rev Cancer 1:214–221 10.1038/35106058 [DOI] [PubMed] [Google Scholar]
- Kilinç MO, Ninis VN, Ugur SA, Tüysüz B, Seven M, Balci S, Goodship J, Tolun A (2003) Is the novel SCKL3 at 14q23 the predominant Seckel locus? Eur J Hum Genet 11:851–857 10.1038/sj.ejhg.5201057 [DOI] [PubMed] [Google Scholar]
- Koury MJ, Park DJ, Martincic D, Horne DW, Kravtsov V, Whitlock JA, del Pilar Aguinaga M, Kopsombut P (1997) Folate deficiency delays the onset but increases the incidence of leukemia in Friend virus-infected mice. Blood 90:4054–4061 [PubMed] [Google Scholar]
- Kuo MT, Vyas RC, Jiang LX, Hittelman WN (1994) Chromosome breakage at a major fragile site associated with P-glycoprotein gene amplification in multidrug-resistant CHO cells. Mol Cell Biol 14:5202–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleyman JS (1984) Constitutional hypoplastic anemia associated with familial “bird-headed” dwarfism (Seckel syndrome). Am J Pediatr Hematol Oncol 6:207–209 [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M (2001) The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557–561 10.1038/35087613 [DOI] [PubMed] [Google Scholar]
- Mishmar D, Rahat A, Scherer SW, Nyakatura G, Hinzmann B, Kohwi Y, Mandel-Gutfroint Y, Lee JR, Drescher B, Sas DE, Margalit H, Platzer M, Weiss A, Tsui LC, Rosenthal A, Kerem B (1998) Molecular characterization of a common fragile site (FRA7H) on human chromosome 7 by the cloning of a simian virus 40 integration site. Proc Natl Acad Sci USA 95:8141–8146 10.1073/pnas.95.14.8141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL (2001) ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Natl Acad Sci USA 98:9092–9097 10.1073/pnas.161281798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33:497–501 10.1038/ng1129 [DOI] [PubMed] [Google Scholar]
- Popescu NC, DiPaolo JA (1989) Preferential sites for viral integration on mammalian genome. Cancer Genet Cytogenet 42:157–171 10.1016/0165-4608(89)90084-8 [DOI] [PubMed] [Google Scholar]
- Rassool FV, McKeithan TW, Neilly ME, van Melle E, Espinosa III R, Le Beau MM (1991) Preferential integration of marker DNA into the chromosomal fragile site at 3p14: an approach to cloning fragile sites. Proc Natl Acad Sci USA 88:6657–6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckel HPG (1960) Bird-headed Dwarfs: studies in developmental anthropology including human proportions. C. C. Thomas, Springfield, IL [Google Scholar]
- Smith PP, Friedman C, Bryant EM, McDougall JK (1992) Viral integration and fragile sites in human papillomavirus-immortalized human keratinocyte cell lines. Genes Chromosomes Cancer 5:150–157 [DOI] [PubMed] [Google Scholar]
- Syrrou M, Georgiou I, Paschopoulos M, Lolis D (1995) Seckel syndrome in a family with three affected children and hematological manifestations associated with chromosome instability. Genet Couns 6:37–41 [PubMed] [Google Scholar]
- Tercero JA, Diffley JFX (2001) Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412:553–557 10.1038/35087607 [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Stroes E, Rabelink TJ (2002) Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol 22:6–13 10.1161/hq0102.102190 [DOI] [PubMed] [Google Scholar]
- Wilke CM, Hall BK, Hoge A, Paradee W, Smith DI, Glover TW (1996) FRA3B extends over a broad region and contains a spontaneous HPV16 integration site: direct evidence for the coincidence of viral integration sites and fragile sites. Hum Molec Genet 5:187–195 10.1093/hmg/5.2.187 [DOI] [PubMed] [Google Scholar]
- Woods CG, Leversha M, Rogers JG (1995) Severe intrauterine growth retardation with increased mitomycin C sensitivity: a further chromosome breakage syndrome. J Med Genet 32:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]