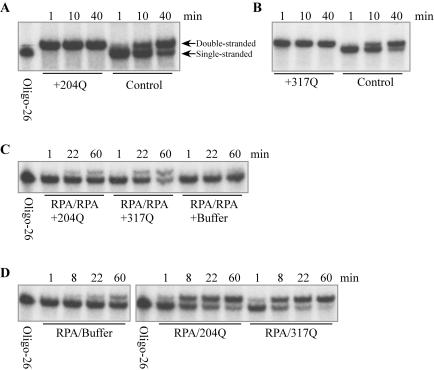
Figure 4.
Annealing assays in vitro. (A and B) Annealing of oligonucleotides is accelerated by truncated Rad52 proteins. Labeled oligo-26 (125 fmol) and 100 pmol truncated Rad52 protein were mixed in 50 μl RPA buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM DTT and 0.02% Triton X-100) and incubated at 22°C for 20 min. Then, 125 fmol unlabeled oligo-25 was added to initiate annealing. At various times, 10 μl was removed and 50 pmol of unlabeled oligo-26 was added to stop annealing. Samples were treated with 0.5% SDS and 0.1 mg/ml proteinase K for 15 min at 37°C, and analyzed by 10% PAGE. The type of truncated Rad52 that was used is indicated; controls contained buffer in place of Rad52. (C) Rad52 1–317Q NLS mediates annealing when both strands are complexed by RPA. Labeled oligo-26 and unlabeled oligo-25 (188 fmol each) were preincubated separately with 260 pmol RPA in 75 μl RPA buffer for 20 min at 22°C, and then the samples were mixed. Aliquots (48 μl) of this mixture were incubated with 140 pmol of either Rad52 1–204Q NLS or Rad52 1–317Q NLS proteins. At various times, the reaction was stopped and analyzed by PAGE as described above. (D) Both Rad52 1–204Q NLS and Rad52 1–317Q NLS mediate annealing when RPA is bound to one strand. Unlabeled oligo-25 (375 fmol) was preincubated with 510 pmol RPA in 150 μl RPA buffer for 20 min at 22°C, while 125 fmol labeled oligo-26 was preincubated separately with 1.8 nmol Rad52 1–204Q NLS or Rad52 1–317Q NLS in 50 μl RPA buffer. Fifty microliters of the oligo-25+RPA sample was mixed with one of the oligo-26+Rad52 samples, incubated at 22°C and analyzed as described above.