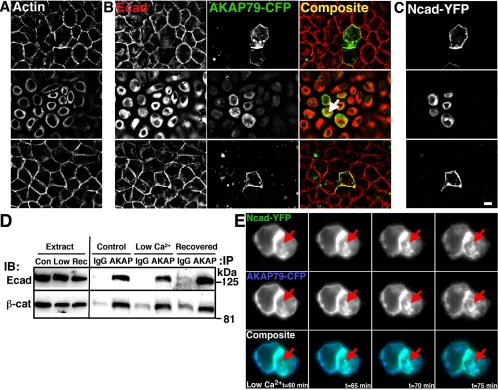
Figure 8.
Disruption of cadherin homophilic interactions by low extracellular Ca2+ leads to loss of AKAP79 lateral membrane localization in epithelial cells. (A–C) MDCK cells transfected with AKAP79-CFP and Ncad-YFP were imaged under control conditions (top row, 2 mM Ca2+), in reduced calcium (middle row, 2 μM Ca2+, 1 h), and after recovery (bottom row, 2 mM Ca2+, 1 h). (A) F-actin (monochrome, not in composite) lateral membrane localization (control, top row) is lost in low calcium media (middle row) that is restored to pretreatment conditions after 1-h recovery in normal calcium (bottom row). (B) Under control conditions (top row), AKAP79-CFP (green) colocalizes (yellow) with endogenous Ecad (red) at lateral membranes. Despite reduced colocalization of AKAP79-CFP with endogenous Ecad at lateral membranes in low calcium conditions (middle row), some intracellular colocalization is still seen (arrow). AKAP and Ecad lateral membrane localization is restored after 1-h recovery (bottom row). (C) Ncad-YFP (monochrome, not in composite) shows a similar distribution to endogenous Ecad in all experimental conditions. (D) Despite loss of membrane colocalization, inhibition of cadherin adhesion by low Ca2+ does not disrupt AKAP coIP of Ecad or β-cat in approximately 5 mg of AKAP150-transfected MDCK cell lysates. Nonimmune IgG is used as a control for IP specificity. (E) AKAP79-CFP (blue) and Ncad-YFP (green) are lost from lateral membranes and occur in common intracellular structures (arrows) over the same time course in living MDCK cells in low Ca2+ (t = 60–75 min, 33°C). Bar, 10 μm.