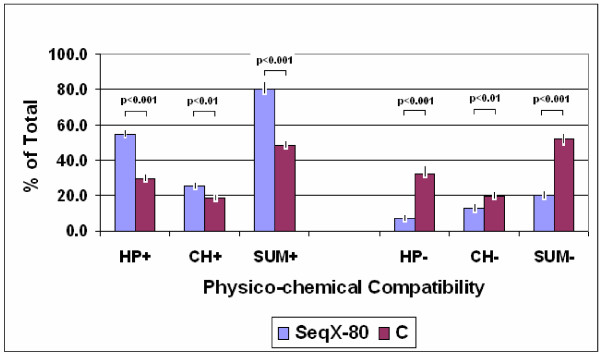
Figure 11.
Real vs. calculated residue co-locations. The relative frequency of real residue co-locations were determined by SeqX in 80 different protein structures and compared to the relative frequency of calculated co-locations in artificial, random protein sequences (C). The 200 possible residue pairs provided by the 20 amino acids were grouped into 4 subgroups regarding their physico-chemical compatibility to each other i.e. favored (+) and un-favored (-) regarding hydrophobicity and charge. (HP+: hydrophobe – hydrophobe and lipophobe – lipophobe; HP-: hydrophobe – lipophobe; CH+: positive – negative and hydrophobe – charged; CH-: positive-positive and negative -negative and lipophobe – charged interactions). The bars represent the mean +/- S.E.M. (n = 80 for real structures and n = 10 for artificial sequences). Student's t-test was applied to evaluate the results.