Fig. 1.
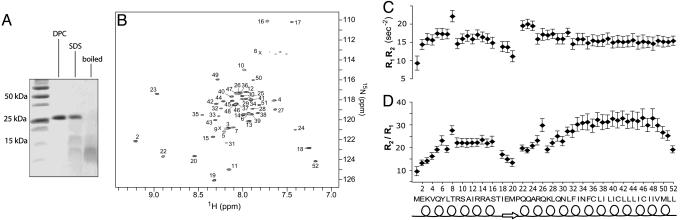
NMR characterization of PLN pentamer. (A) SDS/PAGE of PLN reconstituted in 200 mM DPC and SDS under reducing conditions. Lane 1 shows the molecular mass marker. In lanes 2 and 3, PLN was reconstituted in DPC and SDS, respectively, and was loaded on the gel without boiling. For lane 4, the DPC-reconstituted sample was boiled for 30 min before loading and pentamers were disrupted. The PLN concentration (0.02 mM) is the same in lanes 2-4. (B) A 750-MHz 1H-15N transverse relaxation-optimized spectroscopy spectrum of 0.2 mM PLN pentamer in DPC. Each peak represents a backbone NH moiety with residue number labeled. The C5 rotational symmetry is manifested in the presence of one set of 50 amide peaks. (C) The products of 15N longitudinal (R1) and transverse (R2) relaxation rates used to probe variations in chemical exchange (26); the rates were recorded at 1H frequency of 600 MHz by using experiments that closely followed that of Farrow et al. (45). (D) The different ratios of R2 to R1 for AP and TM helices indicate that the AP helices are mobile relative to the TM helices in the pentamer.