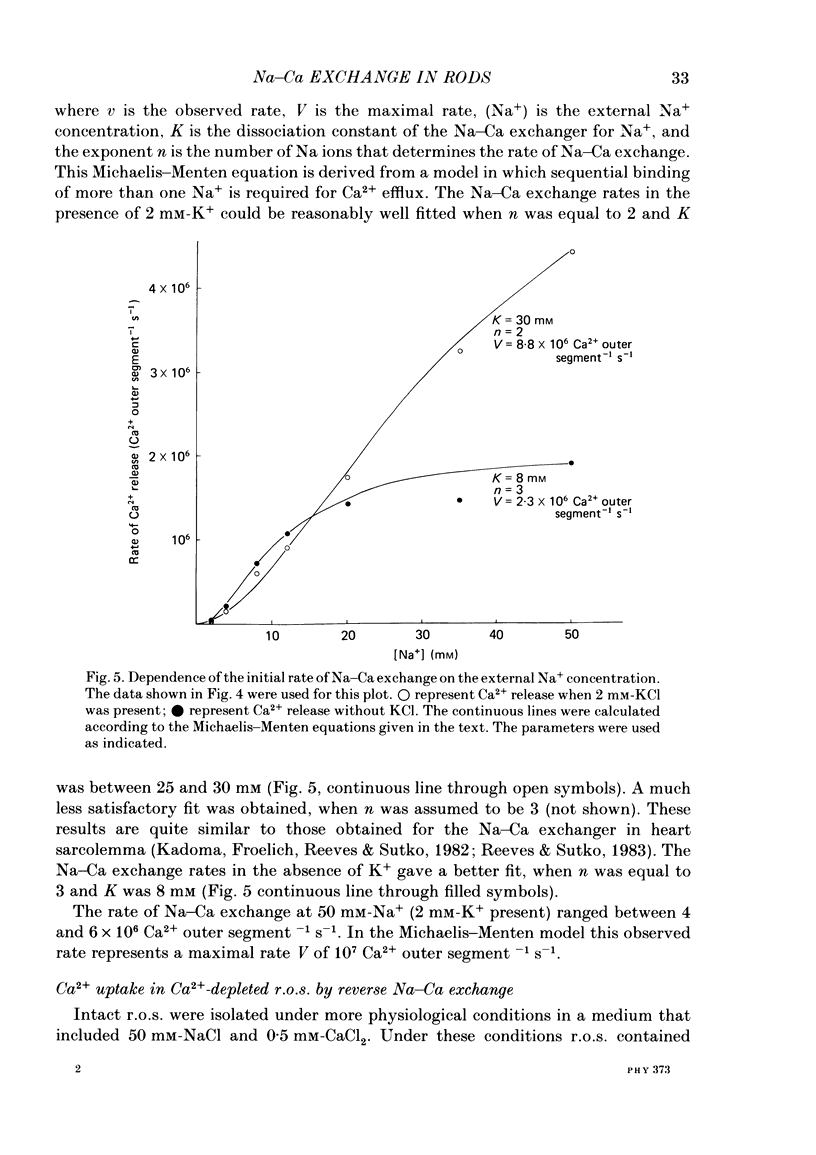
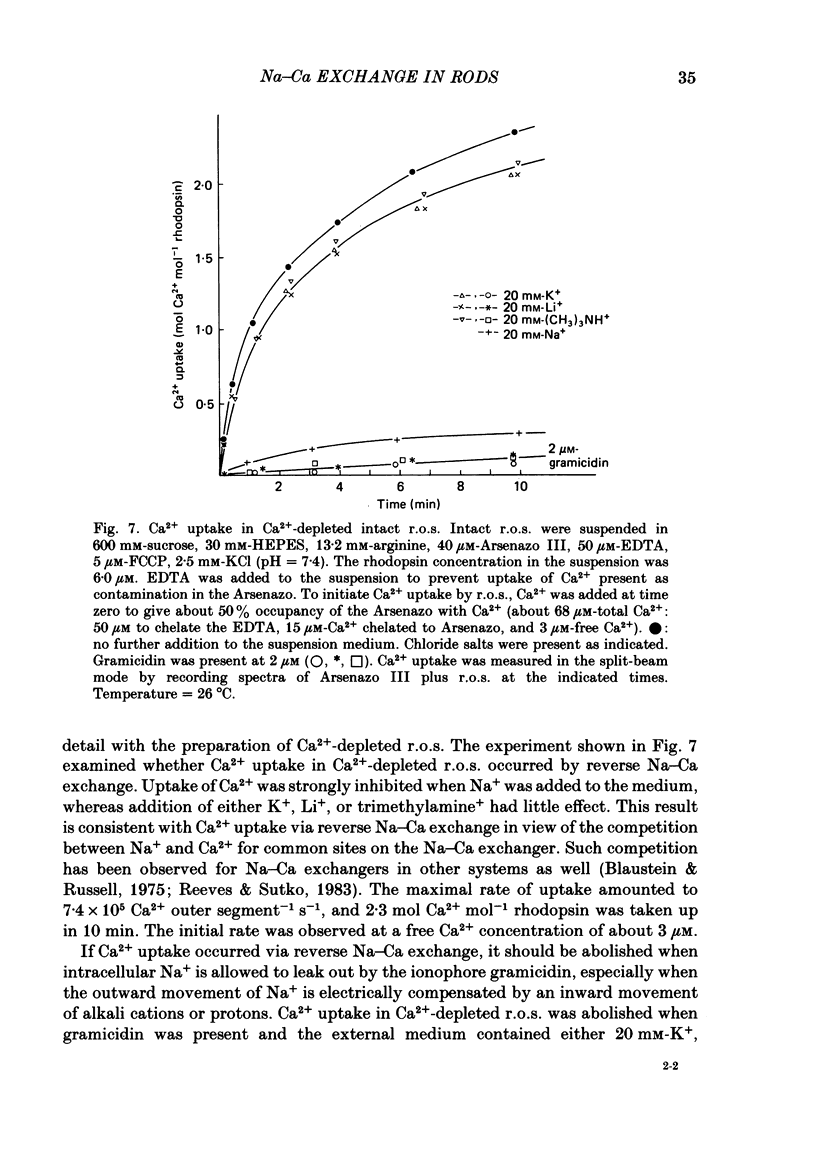
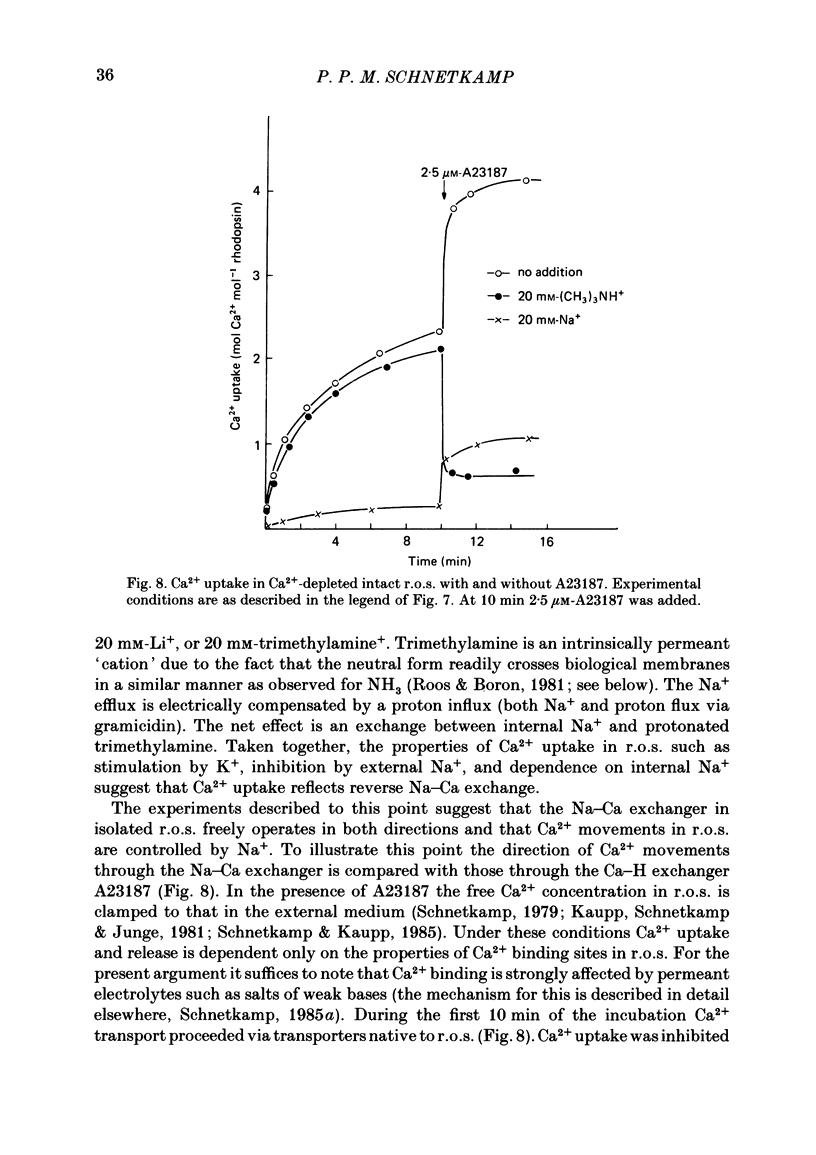
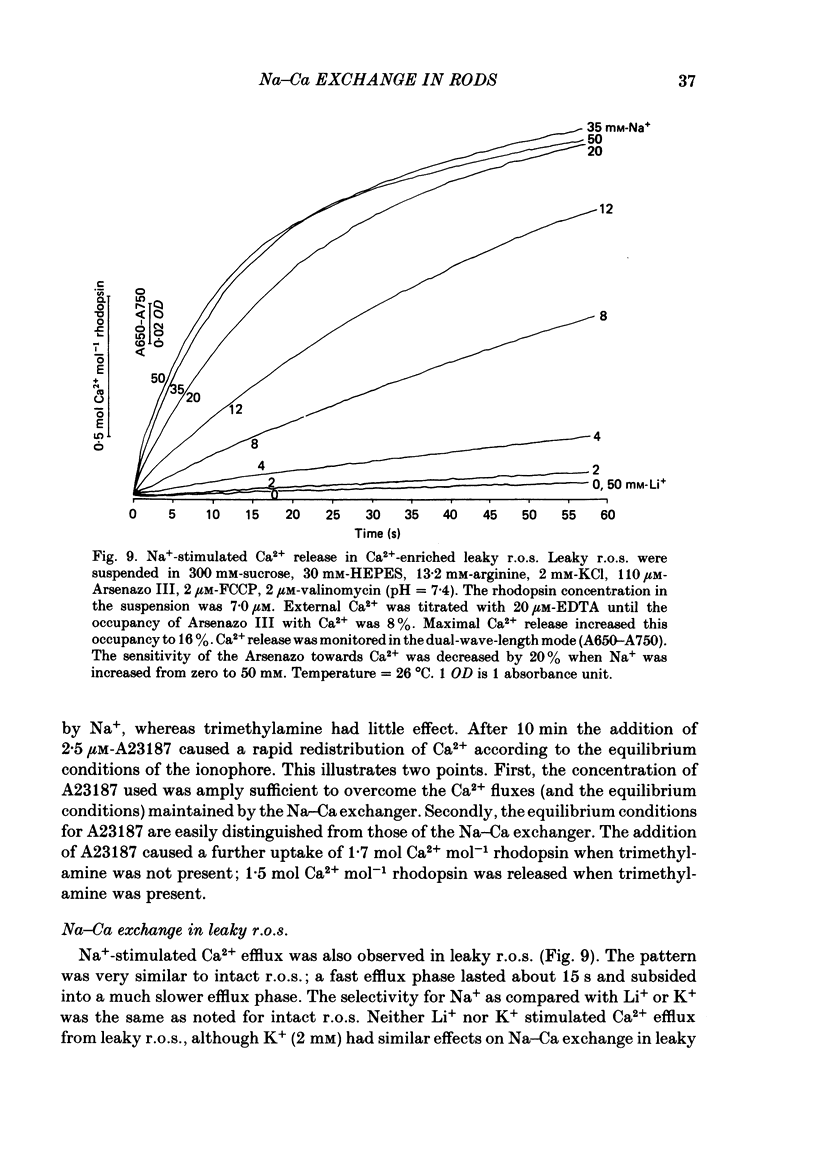
Abstract
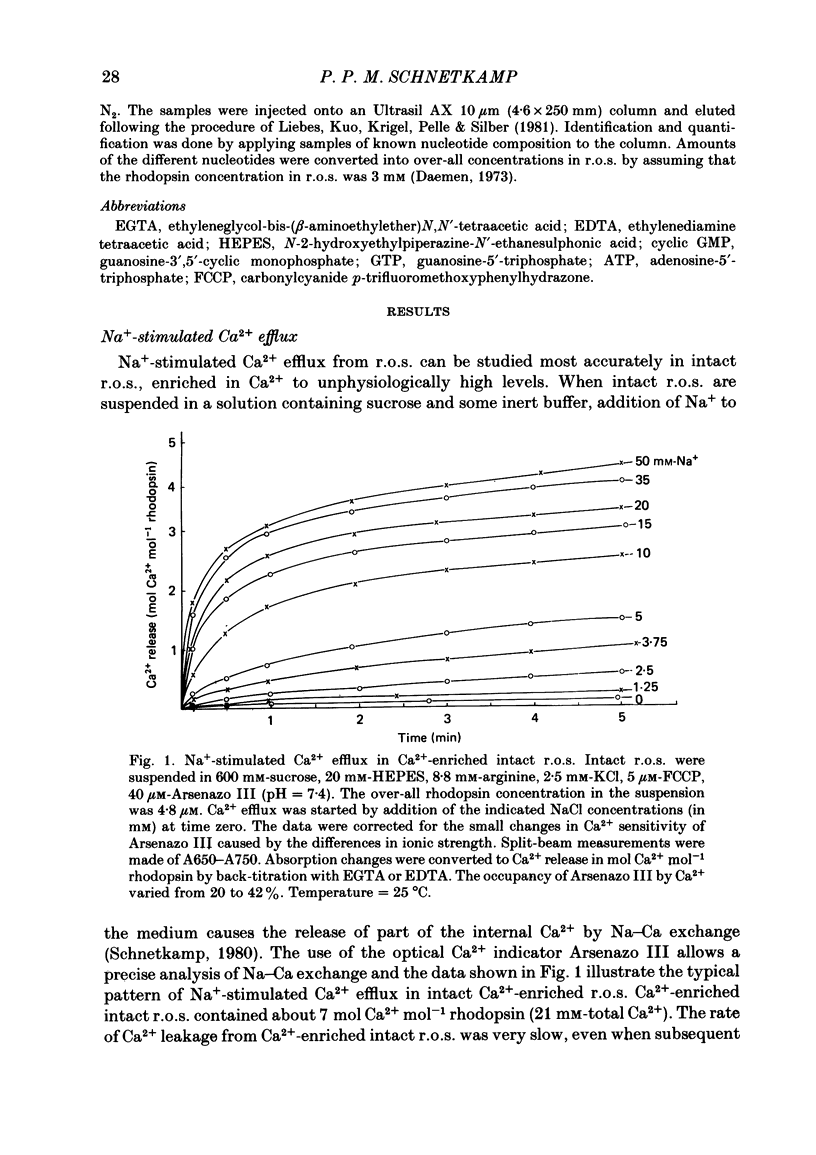
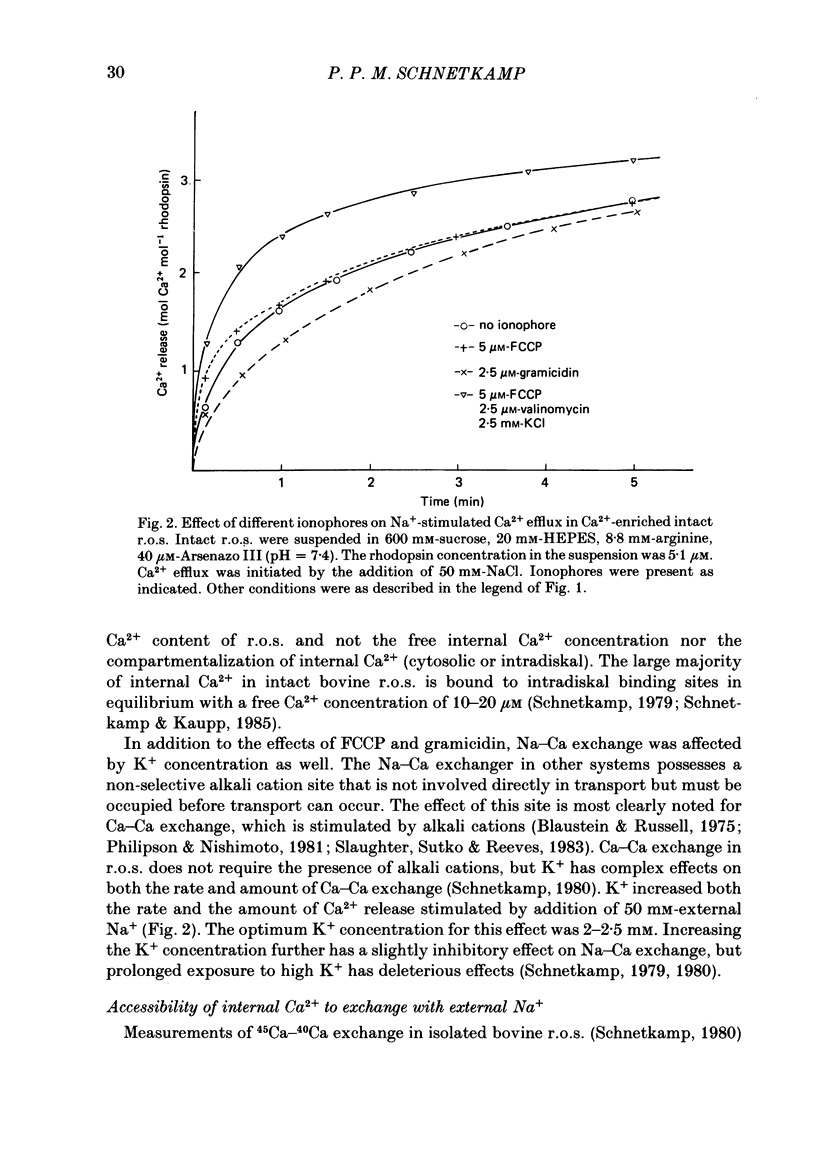
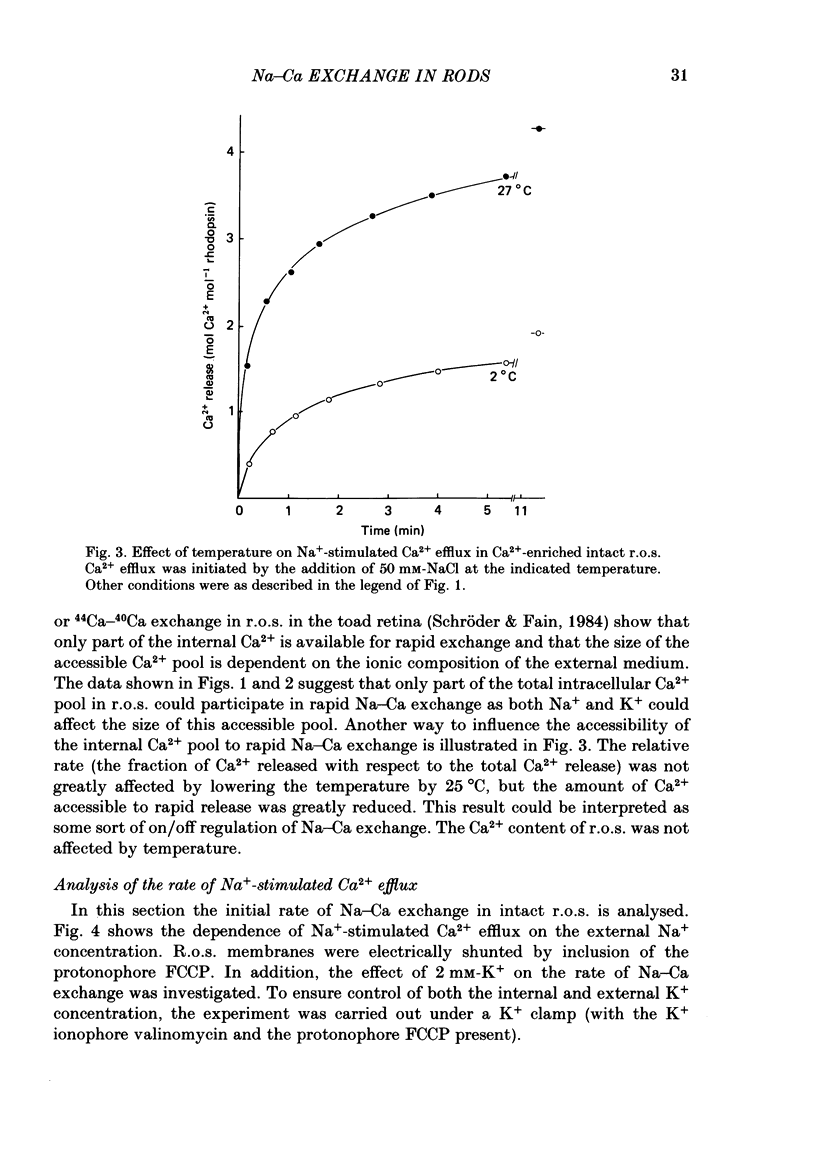
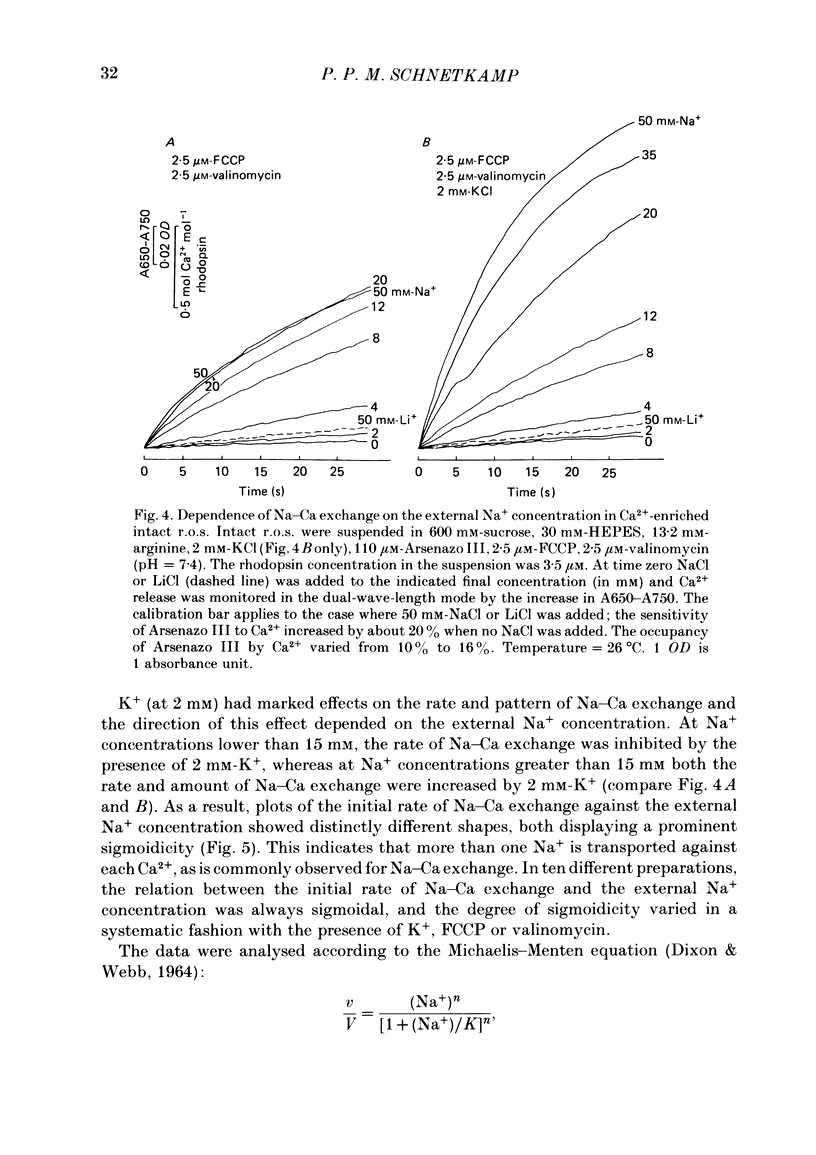
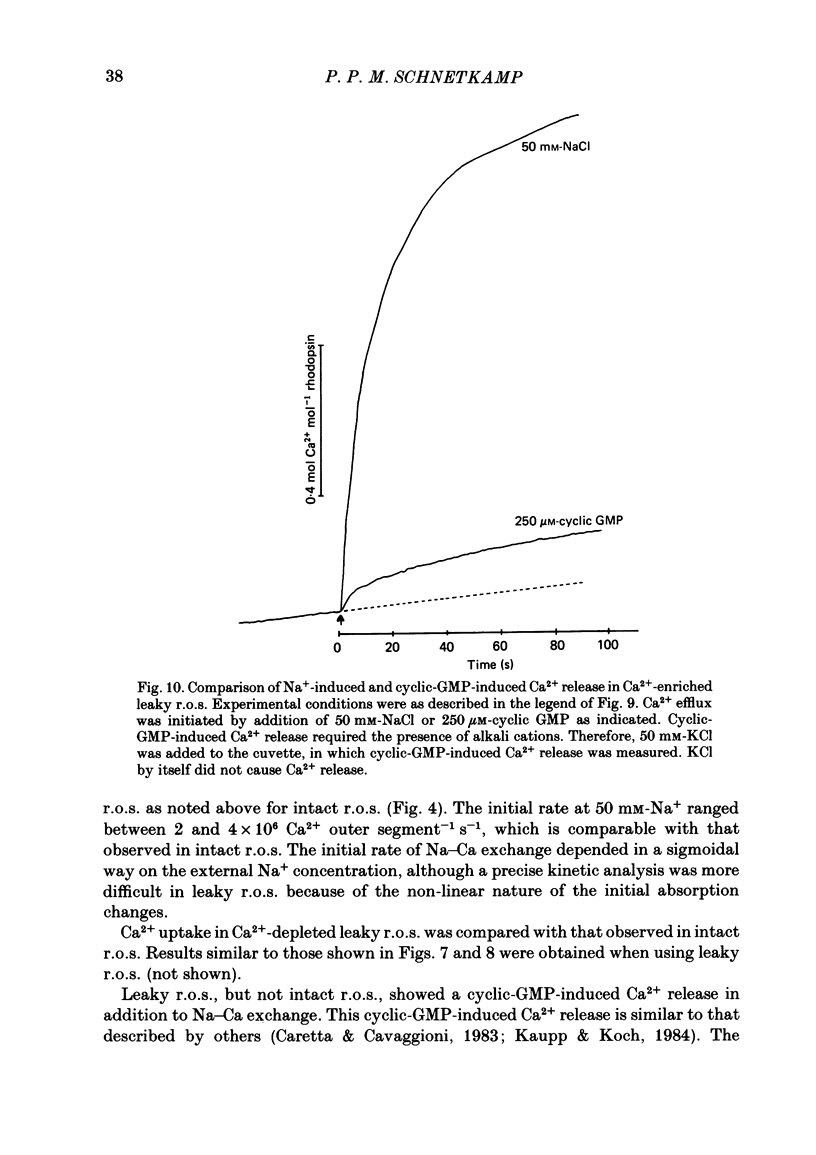
Intact rod outer segments (r.o.s.) isolated from bovine retinas were used to measure net Ca2+ fluxes using the optical Ca2+ indicator Arsenazo III. Ca2+ fluxes were observed, which could change the internal Ca2+ content of isolated r.o.s. by as much as 0.5 mM s-1. The Ca2+ content of isolated intact r.o.s. was strongly dependent on the Na/Ca ratio in the isolation medium, and could be made less than 0.1 mol Ca2+ mol-1 rhodopsin (zero Ca2+ in isolation medium) or up to 7 mol Ca2+ mol-1 rhodopsin (zero Na+ in isolation medium). Ca2+ efflux from r.o.s. rich in Ca2+ was observed only when Na+ was added to the external medium (as opposed to any other alkali cation); in Ca2+-depleted r.o.s. Ca2+ uptake required the presence of internal Na+ and was inhibited selectively by external Na+. These results suggest that Na-Ca exchange across the plasma membrane operated freely in both directions and controlled the internal Ca2+ concentration in r.o.s. Na+-stimulated Ca2+ efflux depended on the external Na+ concentration in a sigmoidal way. This suggests that the simultaneous binding of two Na ions is rate limiting for transport. In Ca2+-depleted r.o.s. and in the absence of external Na+, 1 mol Ca2+ mol-1 rhodopsin (or 3 mM-total Ca2+) could be taken up within 1 min by intact r.o.s. at a free external Ca2+ concentration of about 1 microM. Only part of the internal Ca2+ was available for Na-Ca exchange. The external Na+ and K+ concentration as well as the temperature were factors controlling the accessibility of internal Ca2+ to participate in Na-Ca exchange. Ca2+ fluxes in r.o.s. with a permeabilized plasma membrane but intact disk membranes were very similar to those observed in intact r.o.s.; Na-Ca exchange could operate in both directions across the disk membrane. In addition to Na-Ca exchange, leaky r.o.s. also showed a guanosine 3', 5'-cyclic monophosphate (cyclic GMP)-induced Ca2+ release that was about 1/20 of the rate of Na-Ca exchange. Na-Ca exchange could release 1.5 mol Ca2+ mol-1 rhodopsin from disks as compared with a cyclic-GMP-induced release of 0.15 mol Ca2+ mol-1 rhodopsin.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of sodium replacement on the responses of toad rods. J Physiol. 1982 Sep;330:331–347. doi: 10.1113/jphysiol.1982.sp014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J. 1983 Mar;41(3):381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. J., DeVries G. W., Carter J. G., Schulz D. W., Passonneau P. N., Lowry O. H., Ferrendelli J. A. The distribution of the components of the cyclic GMP cycle in retina. J Biol Chem. 1980 Apr 10;255(7):3128–3133. [PubMed] [Google Scholar]
- Biernbaum M. S., Bownds M. D. Frog rod outer segments with attached inner segment ellipsoids as an in vitro model for photoreceptors on the retina. J Gen Physiol. 1985 Jan;85(1):83–105. doi: 10.1085/jgp.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A. Fast ionic flux activated by cyclic GMP in the membrane of cattle rod outer segments. Eur J Biochem. 1983 Apr 15;132(1):1–8. doi: 10.1111/j.1432-1033.1983.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Daemen F. J. Vertebrate rod outer segment membranes. Biochim Biophys Acta. 1973 Nov 28;300(3):255–288. doi: 10.1016/0304-4157(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoma M., Froehlich J., Reeves J., Sutko J. Kinetics of sodium ion induced calcium ion release in calcium ion loaded cardiac sarcolemmal vesicles: determination of initial velocities by stopped-flow spectrophotometry. Biochemistry. 1982 Apr 13;21(8):1914–1918. doi: 10.1021/bi00537a033. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Koch K. W. Cyclic GMP releases calcium from leaky rod outer segments. Vision Res. 1984;24(11):1477–1479. doi: 10.1016/0042-6989(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P. Calcium metabolism in vertebrate photoreceptors. Cell Calcium. 1982 May;3(2):83–112. doi: 10.1016/0143-4160(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P., Junge W. Rapid calcium release and proton uptake at the disk membrane of isolated cattle rod outer segments. 1. Stoichiometry of light-stimulated calcium release and proton uptake. Biochemistry. 1981 Sep 15;20(19):5500–5510. doi: 10.1021/bi00522a024. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I. Signal mechanisms of phototransduction in retinal rod. CRC Crit Rev Biochem. 1985;17(3):223–256. doi: 10.3109/10409238509113605. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebes L. F., Kuo S., Krigel R., Pelle E., Silber R. Identification and quantitation of ascorbic acid in extracts of human lymphocytes by high-performance liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):53–57. doi: 10.1016/0003-2697(81)90155-x. [DOI] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nishimoto A. Y. Efflux of Ca2+ from cardiac sarcolemmal vesicles. Influence of external Ca2+ and Na+. J Biol Chem. 1981 Apr 25;256(8):3698–3702. [PubMed] [Google Scholar]
- Philipson K. D., Nishimoto A. Y. Na+-Ca2+ exchange is affected by membrane potential in cardiac sarcolemmal vesicles. J Biol Chem. 1980 Jul 25;255(14):6880–6882. [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Reeves J. P., Hale C. C. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem. 1984 Jun 25;259(12):7733–7739. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Competitive interactions of sodium and calcium with the sodium-calcium exchange system of cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3178–3182. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Sodium-calcium exchange activity generates a current in cardiac membrane vesicles. Science. 1980 Jun 27;208(4451):1461–1464. doi: 10.1126/science.7384788. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochim Biophys Acta. 1979 Jul 5;554(2):441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XXXVI. Calcium accumulation in cattle rod outer segments: evidence for a calcium-sodium exchange carrier in the rod sac membrane. Biochim Biophys Acta. 1977 Jul 14;468(2):259–270. doi: 10.1016/0005-2736(77)90119-5. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Daemen F. J. Isolation and characterization of osmotically sealed bovine rod outer segments. Methods Enzymol. 1982;81:110–116. doi: 10.1016/s0076-6879(82)81019-7. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Ion selectivity of the cation transport system of isolated intact cattle rod outer segments: evidence for a direct communication between the rod plasma membrane and the rod disk membranes. Biochim Biophys Acta. 1980 May 8;598(1):66–90. doi: 10.1016/0005-2736(80)90266-7. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Kaupp U. B. Calcium-hydrogen exchange in isolated bovine rod outer segments. Biochemistry. 1985 Jan 29;24(3):723–727. doi: 10.1021/bi00324a028. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Klompmakers A. A., Daemen F. J. The isolation of stable cattle rod outer segments with an intact plasma membrane. Biochim Biophys Acta. 1979 Apr 19;552(3):379–389. doi: 10.1016/0005-2736(79)90182-2. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Metabolism in the cytosol of intact isolated cattle rod outer segments as indicator for cytosolic calcium and magnesium ions. Biochemistry. 1981 Apr 28;20(9):2449–2456. doi: 10.1021/bi00512a014. [DOI] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Slaughter R. S., Sutko J. L., Reeves J. P. Equilibrium calcium-calcium exchange in cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3183–3190. [PubMed] [Google Scholar]
- Trosper T. L., Philipson K. D. Effects of divalent and trivalent cations on Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983 May 26;731(1):63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Fain G. L., Bastian B. L. Light-dependent ion influx into toad photoreceptors. J Gen Physiol. 1982 Oct;80(4):517–536. doi: 10.1085/jgp.80.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]


