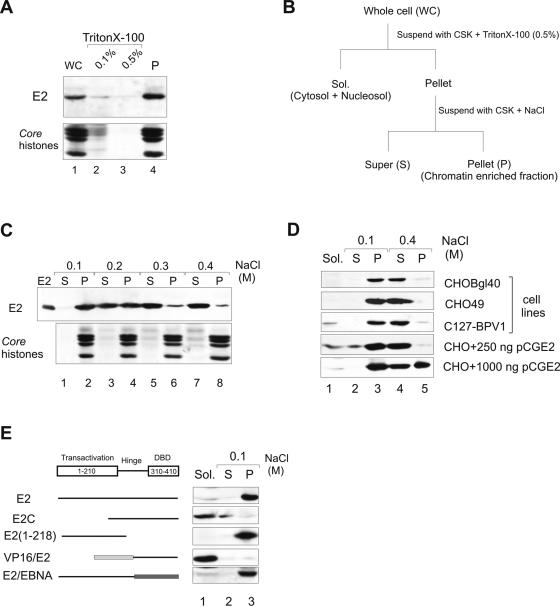
FIG. 1.
Subcellular localization of the BPV1 E2 protein. (A) Association of E2 with a chromatin-containing fraction. CHOBgl40 cells were extracted with Triton X-100 and fractions were analyzed by immunoblotting with E2-specific antibodies. The lower part of the gel was analyzed for the presence of histones (Coomassie blue staining). (B) Protocol for cellular extraction. (C) Salt extractability of E2 expressed by the CHOBgl40 cell line. The chromatin-enriched fraction from panel A was further incubated for 15 min with CSK buffer containing the indicated salt concentrations, separated into soluble (S) and insoluble (P) fractions, and the two fractions were subjected to immunoblotting as in panel A. Proteins from 106 cells were loaded in each lane. (D) Salt extractability of E2 expressed by various cell lines and in CHO cells transiently. For transient expression, two different plasmid concentrations were used and the cells were lysed 24 h after transfection. Cells were extracted as in panel B, and the E2 protein from all fractions of CHO49 and C127-BPV1 cells was immunoprecipitated with E2-specific antibody 3F12 coupled to magnetic beads. Proteins from 106 CHOBgl40 cells, 1.5 × 107 CHO49 cells and 1.5 × 107 C127-BPV1 cells were loaded on S and P lanes, and Sol. fractions contain half of the material of S and P fractions. All fractions were subjected to immunoblotting with E2-specific antibodies. (E) E2 mutant association with a chromatin-containing fraction. CHO cells were transfected with 250 ng of expression plasmid encoding the indicated protein, and cells were lysed 24 h after transfection extracted as in panel B and subjected to immunoblotting with E2-specific antibodies.