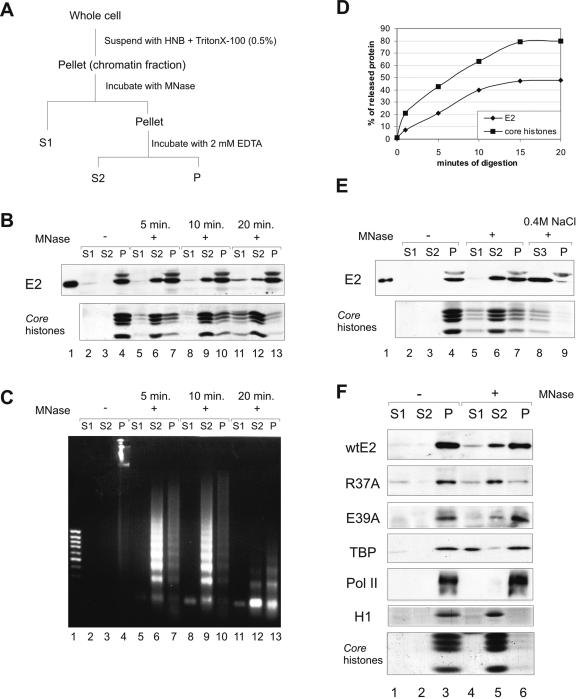
FIG. 5.
The E2 protein on chromatin. (A) Protocol for chromatin fractionation. (B) Nuclei from 2 × 107 CHOBgl40 cells were subjected to partial digestion with 30 U of micrococcal nuclease for the indicated times and separated into supernatant (S1) and pellet. The pellet was extracted with EDTA and centrifuged to yield supernatant (S2) and pellet (P) chromatin fractions. An equal volume of each fraction (extract of 106 cells) was subjected to SDS-PAGE and immunoblotted with E2-specific antibodies; the lower part of the gel was analyzed for the presence of histones (Coomassie blue staining). (C) DNA from each fraction was analyzed by agarose gel electrophoreses and stained with ethidium bromide. Lane 1, 1-kb DNA standard. (D) Mobilization of chromatin bound E2 by MNase. E2 and core histone bands obtained by treatment with 30 U of micrococcal nuclease were quantified by scanning and blotted together as a function of digestion times. The values of two independent experiments are expressed as percentages of total released material. (E) The pellet fraction from panel A was further incubated for 15 min with 0.4 M NaCl and separated into supernatant (S3) and pellet (P). All fractions were subjected to immunoblotting with E2-specific antibodies, and the lower part of the gel was analyzed for the presence of histones. (F) CHO cells were transfected with 200 ng of expression plasmids for E2 and E2 mutants R37A and E39A, and nuclei from 5 × 106 cells were subjected to digestion with 30 U of micrococcal nuclease for 10 min. An equal volume of each fraction was subjected to SDS-PAGE and immunoblotted with E2-specific antibodies and with anti-TBP and anti-Pol II antibodies to detect endogenous proteins; the lower part of the gel was analyzed for the presence of histones (Coomassie blue staining).