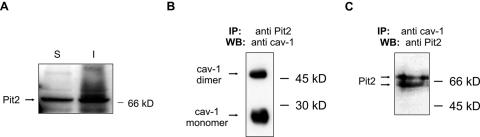
FIG. 4.
Pit2 localization and interaction with caveolin-1. (A) Immunoblot analysis of Triton X-100 soluble (S) and insoluble (I) fractions for the presence of Pit2. NIH 3T3 cells were treated with 0.5% Triton X-100 for 1 min at 4°C. The supernatant containing the soluble fraction and the cell remnant-containing insoluble fraction, respectively, were separated by SDS-PAGE and analyzed by immunoblot for the presence of Pit2 proteins. Similar results were obtained using NIH 3T3 cells overexpressing human Pit2 (not shown). (B) Caveolin-1 coimmunoprecipitate with Pit2. NIH 3T3 cells overexpressing human Pit2 were lysed at room temperature in 1% Triton X-100 and lysates were immunoprecipitated with an anti-Pit2 antibody. The resulting immunoprecipitates were analyzed for the presence of caveolin-1 using SDS-PAGE and immunoblotting. (C) Pit2 coimmunoprecipitate with caveolin-1. NIH 3T3 cells overexpressing human Pit2 were lysed at room temperature in 1% Triton X-100 and lysates were immunoprecipitated with an anti-caveolin-1 antibody. The resulting immunoprecipitates were analyzed for the presence of Pit2 using SDS-PAGE and immunoblotting. IP, immunoprecitation; WB, Western blot (immunoblotting). The anti-Pit2 antibodies recognize both human and mice Pit2. When using an antibody to a multimembrane-spanning protein not harboring a caveolin-binding consensus sequence, no caveolin-1 was coimmunoprecipitated nor could the protein be detected in lysates immunoprecipitated with anti-caveolin-1 antibodies (not shown).