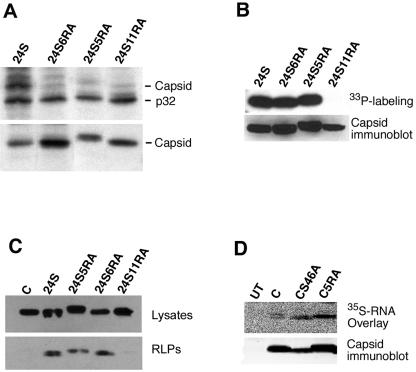
FIG. 6.
Characterization of capsid RA mutants. COS cells were transfected with expression vectors encoding the RV structural proteins (24S) or the RV structural proteins containing arginine-to-alanine mutations in the capsid (24S5RA, 24S6RA, or 24S11RA). (A) Cells were biosynthetically labeled with [35S]methionine-cysteine prior to lysis and immunoprecipitation with either goat anti-p32 serum (upper panel) or rabbit anticapsid serum (lower panel). Samples were subjected to SDS-PAGE and fluorography. The positions of capsid and p32 are indicated to the right of the gels. (B) Cells were labeled with H333PO4, and capsid was immunoprecipitated from lysates. Samples were subjected to SDS-PAGE and fluorography (upper panel). The relative expression levels of capsid were determined by immunoblotting of cell lysates (lower panel). (C) The relative levels of RLP- and cell-associated (Lysate) capsid from transfected cells were determined by immunoblotting. (D) Wild-type (C) and mutant capsid proteins (CS46A and C5RA) were immunoprecipitated from transfected cells, treated with phosphatase, separated by SDS-PAGE, and then transferred to nitrocellulose membranes. Membranes were incubated with 35S-labeled RV genomic RNA and washed, and RNA binding to capsid was detected using a phosphorimager (upper panels). Relative capsid expression levels were determined by stripping the membranes and immunoblotting with a monoclonal antibody to capsid (lower panels). UT, untransfected cells.