Abstract
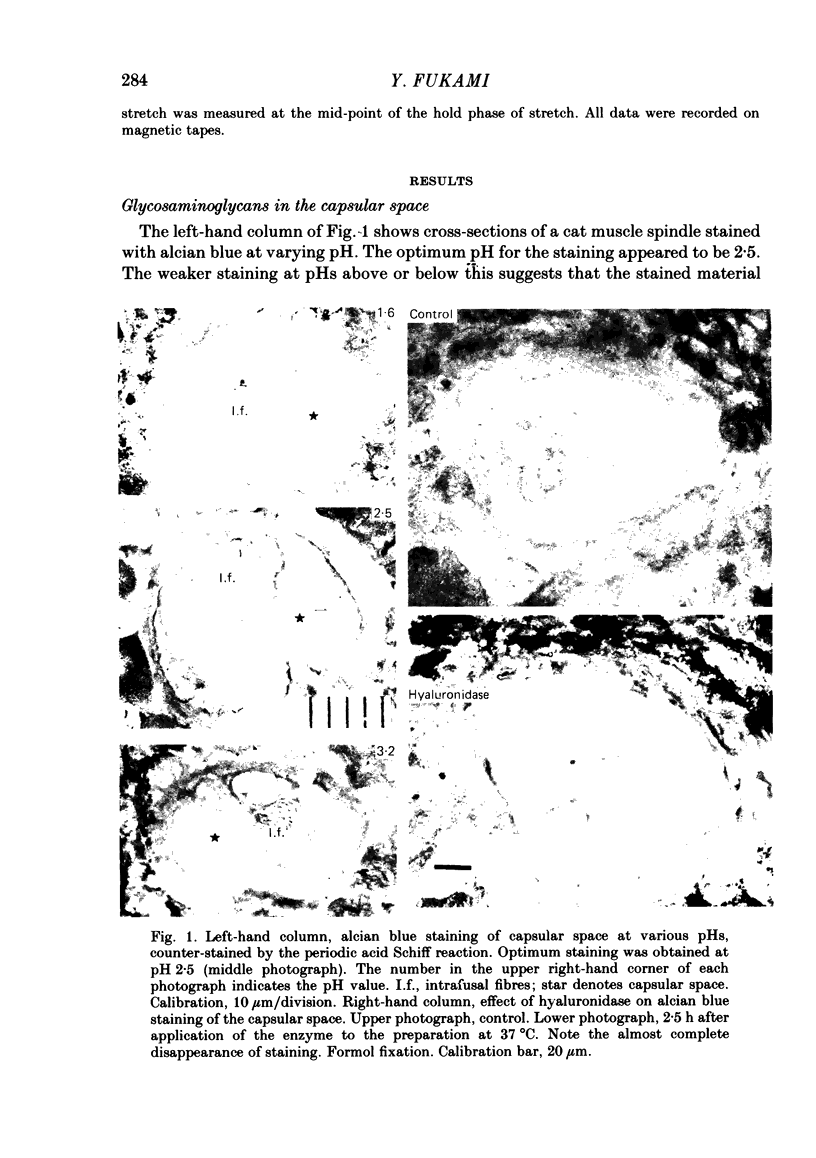
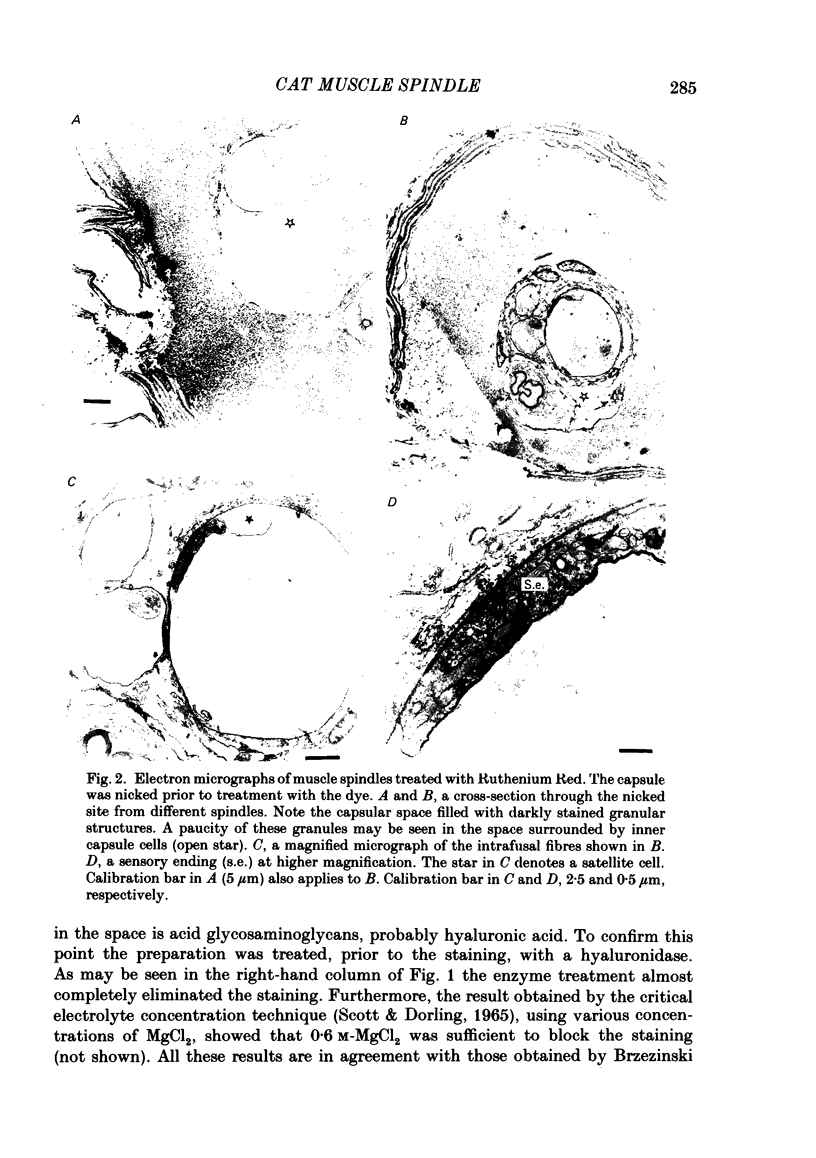
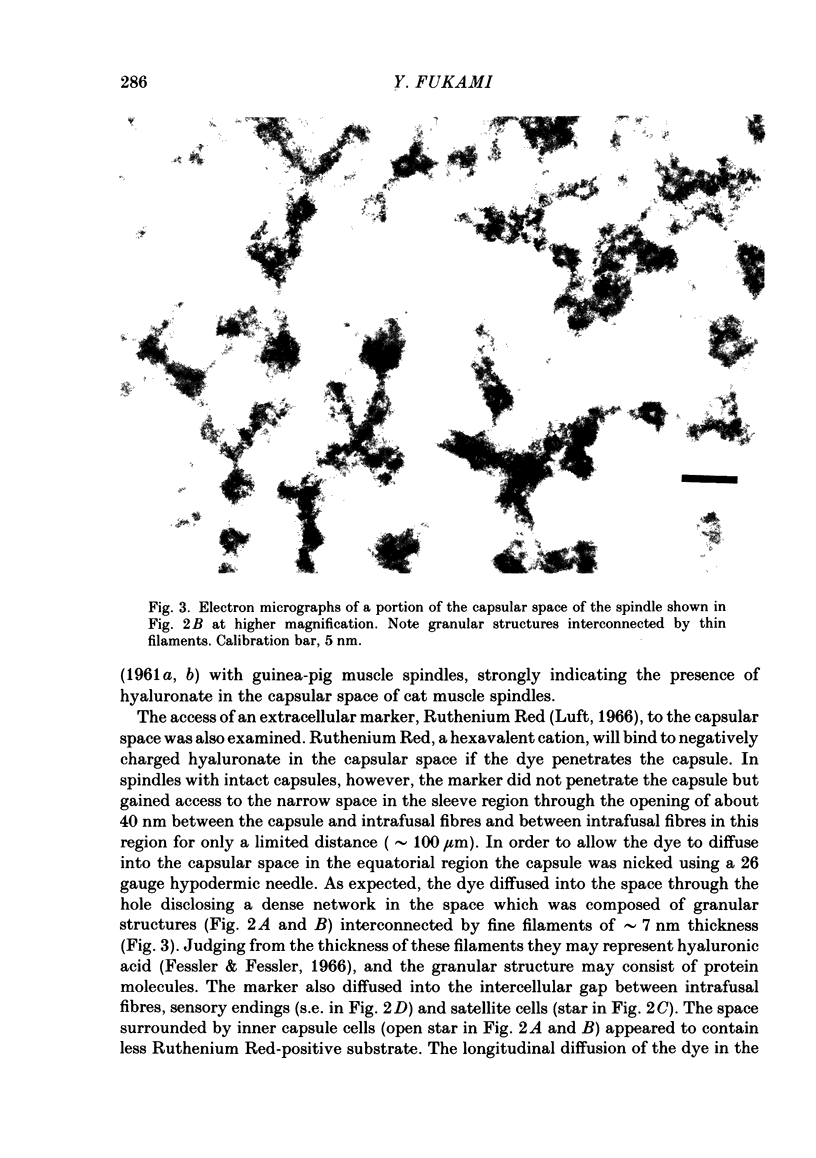
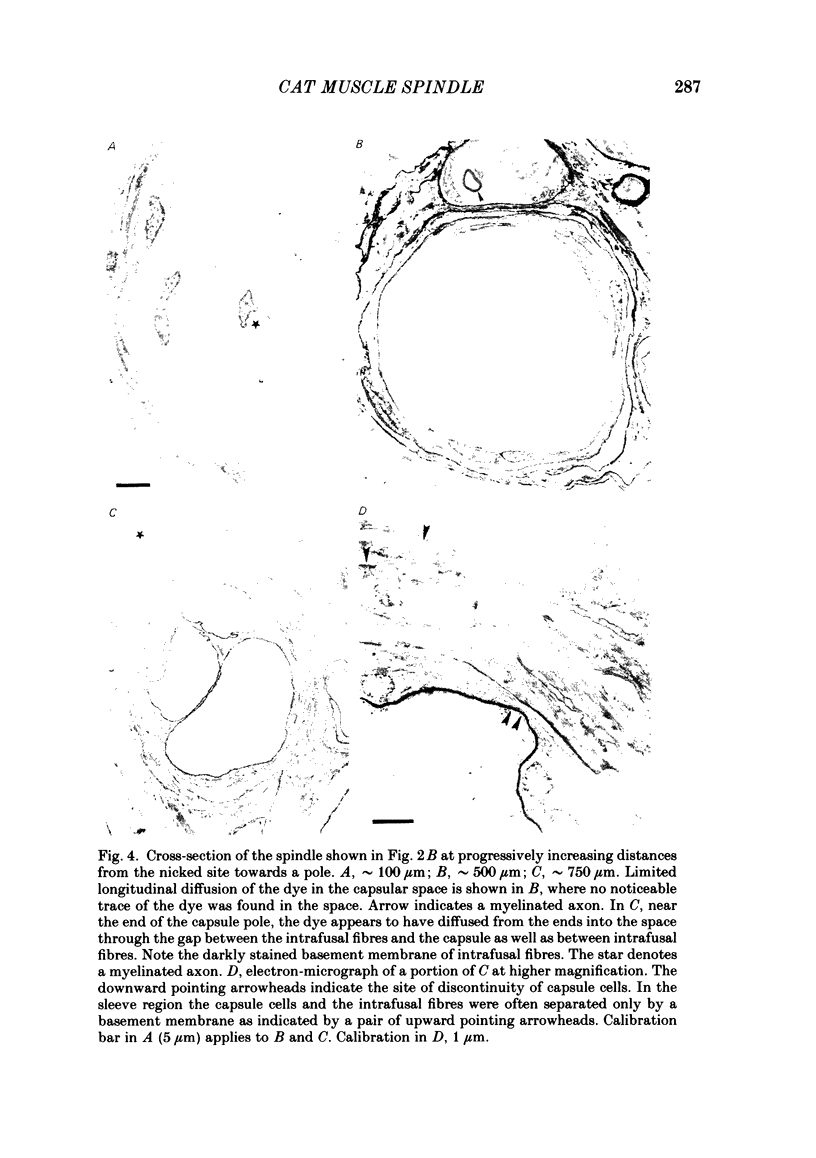
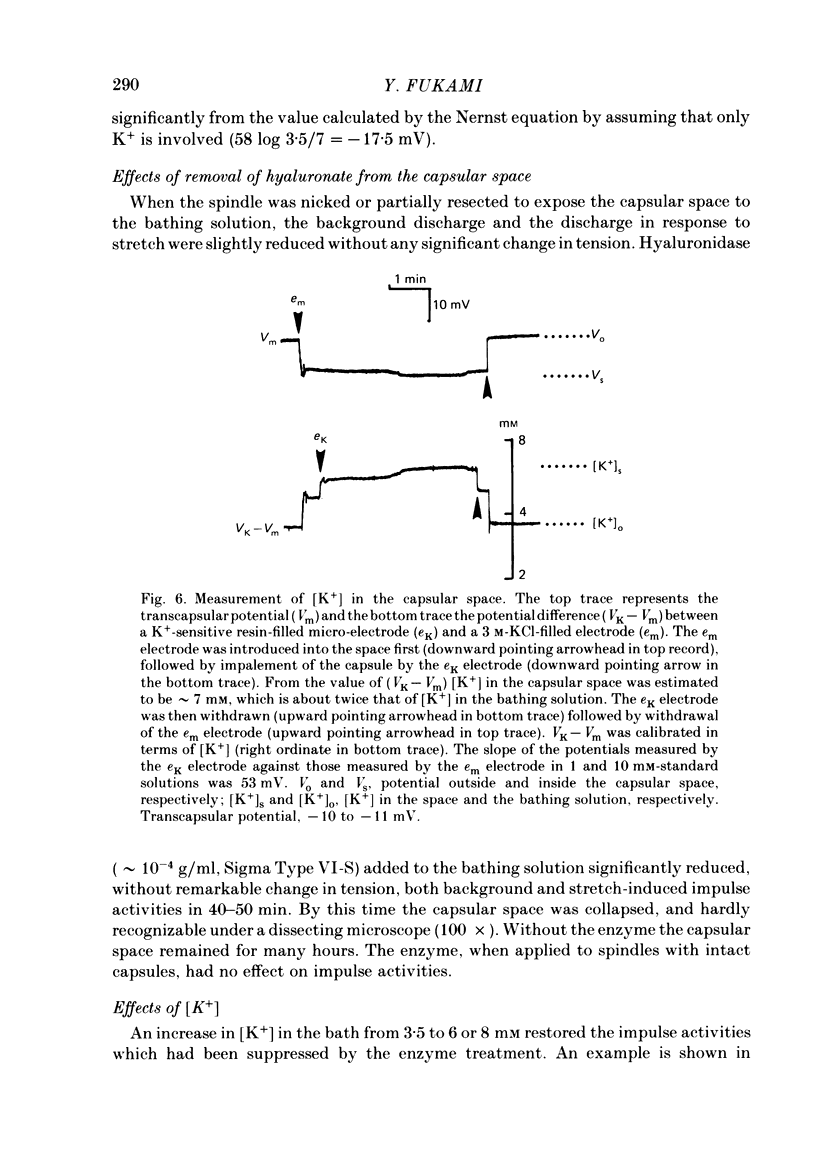
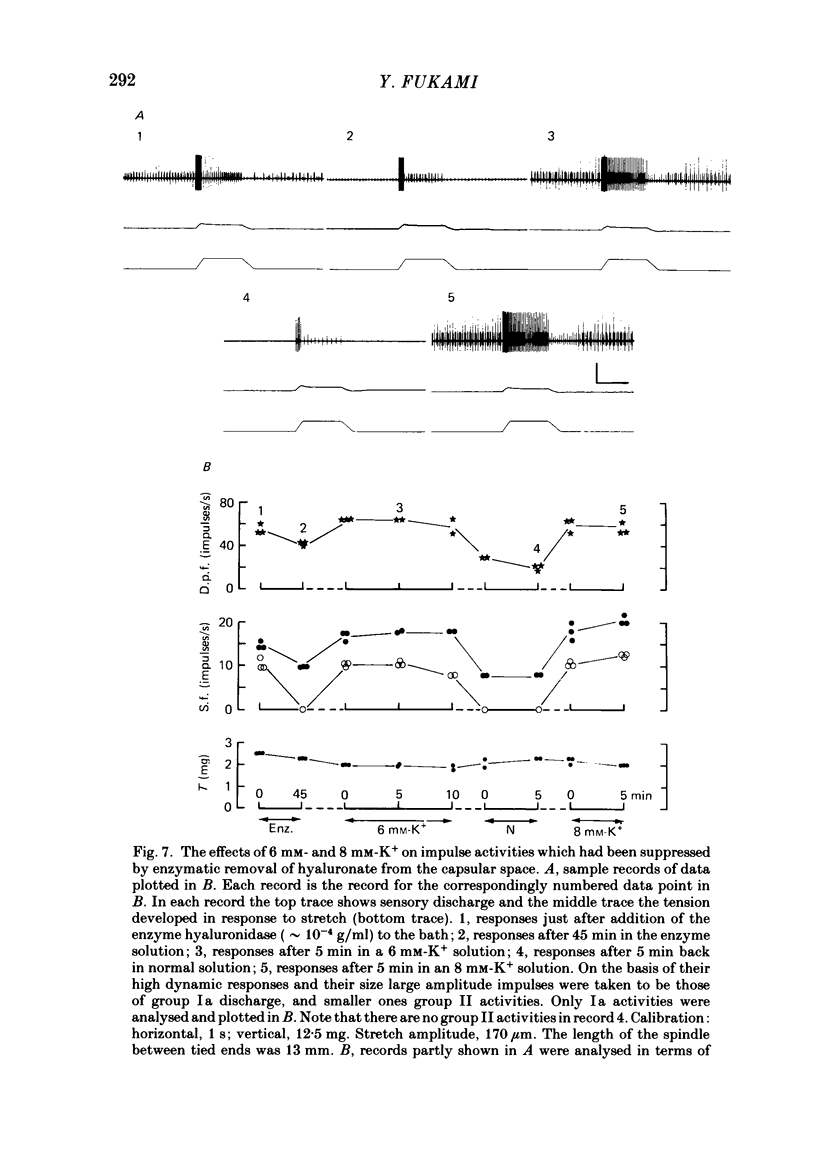
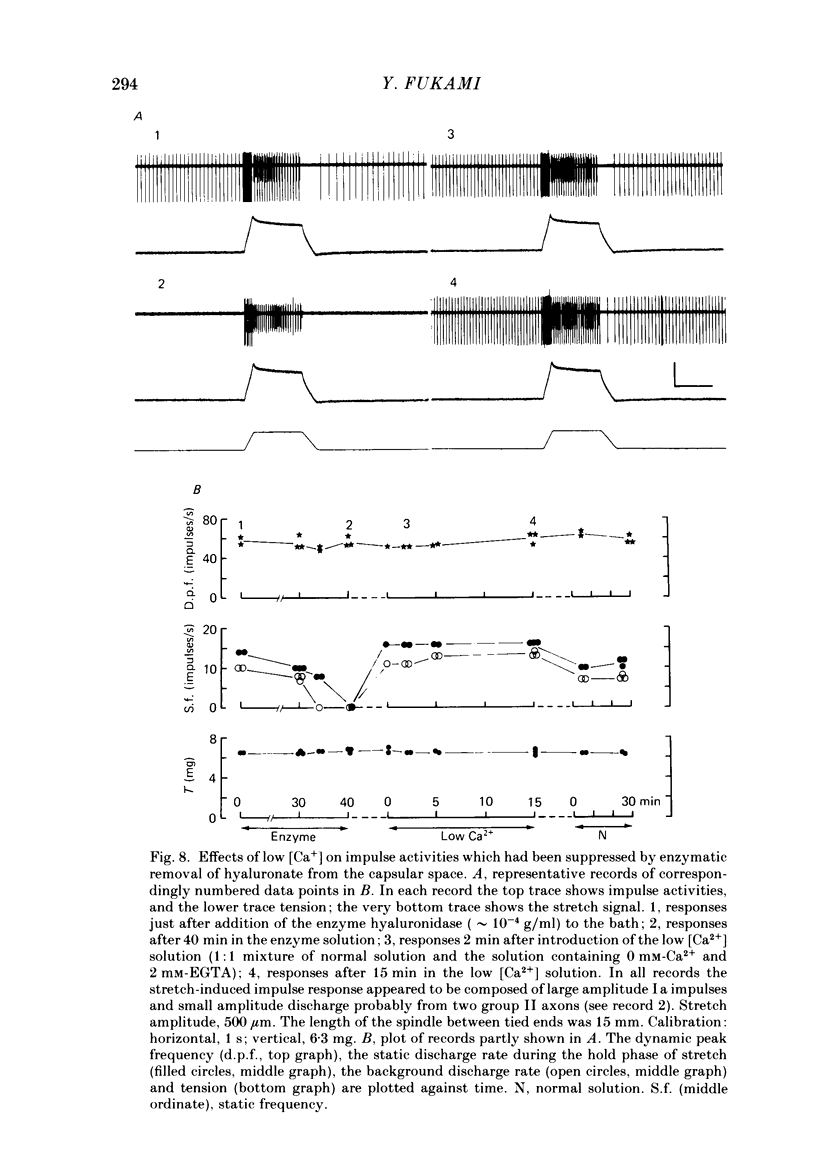
The presence of hyaluronate in the capsular space of the cat muscle spindle was demonstrated using alcian blue staining at various pHs, the critical electrolyte concentration technique and hyaluronidase treatment. In spindles with intact capsules an extracellular marker, the dye Ruthenium Red, gained access to the capsular space through the gap in the sleeve region, but for a limited distance. In muscle spindles with the capsule nicked, the marker diffused into the capsular space in the equatorial region, revealing a dense network in this space which consisted of globular structures interconnected by thin filaments. Based on their thickness, these filaments were inferred to be hyaluronic acid, and the globular structures were inferred to be protein molecules. Longitudinal diffusion of the dye into the capsular space through the nicked site was limited. The limited diffusion is probably due to electrostatic binding of the dye, which is a hexavalent cation, to negatively charged glycosaminoglycan hyaluronate that is present in the space. The transcapsular potential was measured by use of glass micropipettes filled with 3 M-KCl. The value was 15 mV +/- 4 (average +/- S.D., n = 12; range, 10-20 mV) inside negative. The input resistance and capacitance of the capsule, measured with two independent electrodes, varied widely (1.3-8.0 M omega and 0.5-1.3 nF, n = 4) and the capsule showed marked delayed rectification to outward current pulses. [K+] in the space measured with K+-sensitive resin-filled glass micropipettes was a few millimolar higher than that in the bathing solution. The effects of [K+] and [Ca2+] on impulse activities were examined in spindles with intact capsules or with partially resected capsules. In spindles with intact capsules the effects of [K+] and [Ca2+] were significantly less or negligible compared with those in spindles with the capsule opened. Hyaluronidase (approximately 10(-4) g/ml) added to the bathing solution around nicked capsules significantly reduced both resting and stretch-induced impulse activities in 40-50 min. By this time the capsular space was completely collapsed. An increase in [K+] of the bathing solution from 3.5 to 6 or 8 mM restored these impulse activities. A similar restoring effect was also observed when [Ca2+] in the bathing solution was reduced.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIDGMAN C. F., ELDRED E. HYPOTHESIS FOR A PRESSURE-SENSITIVE MECHANISM IN MUSCLE SPINDLES. Science. 1964 Jan 31;143(3605):481–482. doi: 10.1126/science.143.3605.481. [DOI] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Dalgliesh R. J., Mellors L. T., Blight G. W. Comparisons of glucose, sucrose, and dimethyl sulfoxide as cryoprotective agents for Babesia rodhaini, with estimates of survival rates. Cryobiology. 1980 Aug;17(4):410–417. doi: 10.1016/0011-2240(80)90048-6. [DOI] [PubMed] [Google Scholar]
- Dow P. R., Shinn S. L., Ovale W. K., Jr Ultrastructural study of a blood-muscle spindle barrier after systemic administration of horseradish peroxidase. Am J Anat. 1980 Apr;157(4):375–388. doi: 10.1002/aja.1001570406. [DOI] [PubMed] [Google Scholar]
- Fahy G. M. Analysis of "solution effects" injury: rabbit renal cortex frozen in the presence of dimethyl sulfoxide. Cryobiology. 1980 Aug;17(4):371–388. doi: 10.1016/0011-2240(80)90044-9. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Electron microscopic visualization of the polysaccharide hyaluronic acid. Proc Natl Acad Sci U S A. 1966 Jul;56(1):141–147. doi: 10.1073/pnas.56.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami Y. Active force and sensory response of single isolated cat muscle spindles in vitro. J Neurophysiol. 1984 Dec;52(6):1131–1139. doi: 10.1152/jn.1984.52.6.1131. [DOI] [PubMed] [Google Scholar]
- Fukami Y. Further morphological and electrophysiological studies on snake muscle spindles. J Neurophysiol. 1982 May;47(5):810–826. doi: 10.1152/jn.1982.47.5.810. [DOI] [PubMed] [Google Scholar]
- Goldfinger M. D., Fukami Y. Distribution, density and size of muscle receptors in cat tail dorsolateral muscles. J Anat. 1982 Sep;135(Pt 2):371–384. [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Wilkinson R. S. An analysis of receptor potential and tension of isolated cat muscle spindles in response to sinusoidal stretch. J Physiol. 1980 May;302:241–262. doi: 10.1113/jphysiol.1980.sp013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Wilkinson R. S., Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol. 1978 Jun;71(6):683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHN U. Morphological observations on living neuromuscular spindles. Acta Anat (Basel) 1959;39:341–350. doi: 10.1159/000141564. [DOI] [PubMed] [Google Scholar]
- Kennedy W. R., Yoon K. S. Permeability of muscle spindle capillaries and capsule. Muscle Nerve. 1979 Mar-Apr;2(2):101–108. doi: 10.1002/mus.880020204. [DOI] [PubMed] [Google Scholar]
- Kiang W. L., Crockett C. P., Margolis R. K., Margolis R. U. Glycosaminoglycans and glycoproteins associated with microsomal subfractions of brain and liver. Biochemistry. 1978 Sep 5;17(18):3841–3848. doi: 10.1021/bi00611a025. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Reese T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971 Jan;30(1):105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Kennedy W. R., Quick D. C. A determination of static mechanical properties of intrafusal muscle in isolated cat muscle spindles. Neuroscience. 1979;4(3):401–411. doi: 10.1016/0306-4522(79)90103-9. [DOI] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A. Intracellular ionic activities and transmembrane electrochemical potential differences in gallbladder epithelium. J Membr Biol. 1979 Sep 14;49(4):345–362. doi: 10.1007/BF01868991. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Roles of extracellular matrix in neural development. Annu Rev Physiol. 1983;45:581–600. doi: 10.1146/annurev.ph.45.030183.003053. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Shantha T. R., Golarz M. N., Bourne G. H. Histological and histochemical observations on the capsule of the muscle spindle in normal and denervated muscle. Acta Anat (Basel) 1968;69(4):632–646. doi: 10.1159/000143103. [DOI] [PubMed] [Google Scholar]
- VOSS H. [An unusual and abundant presence of free cells in the capsular space of muscle spindles in the newborn infant]. Anat Anz. 1962 Feb 15;110:417–419. [PubMed] [Google Scholar]
- Wilkinson R. S., Fukami Y. Responses of isolated Golgi tendon organs of cat to sinusoidal stretch. J Neurophysiol. 1983 Apr;49(4):976–988. doi: 10.1152/jn.1983.49.4.976. [DOI] [PubMed] [Google Scholar]
- von BRZEZINSKI D. [Studies on the histochemistry of muscle spindles. I. Topochemistry of polysaccharides]. Acta Histochem. 1961 Oct 20;12:75–79. [PubMed] [Google Scholar]