Abstract
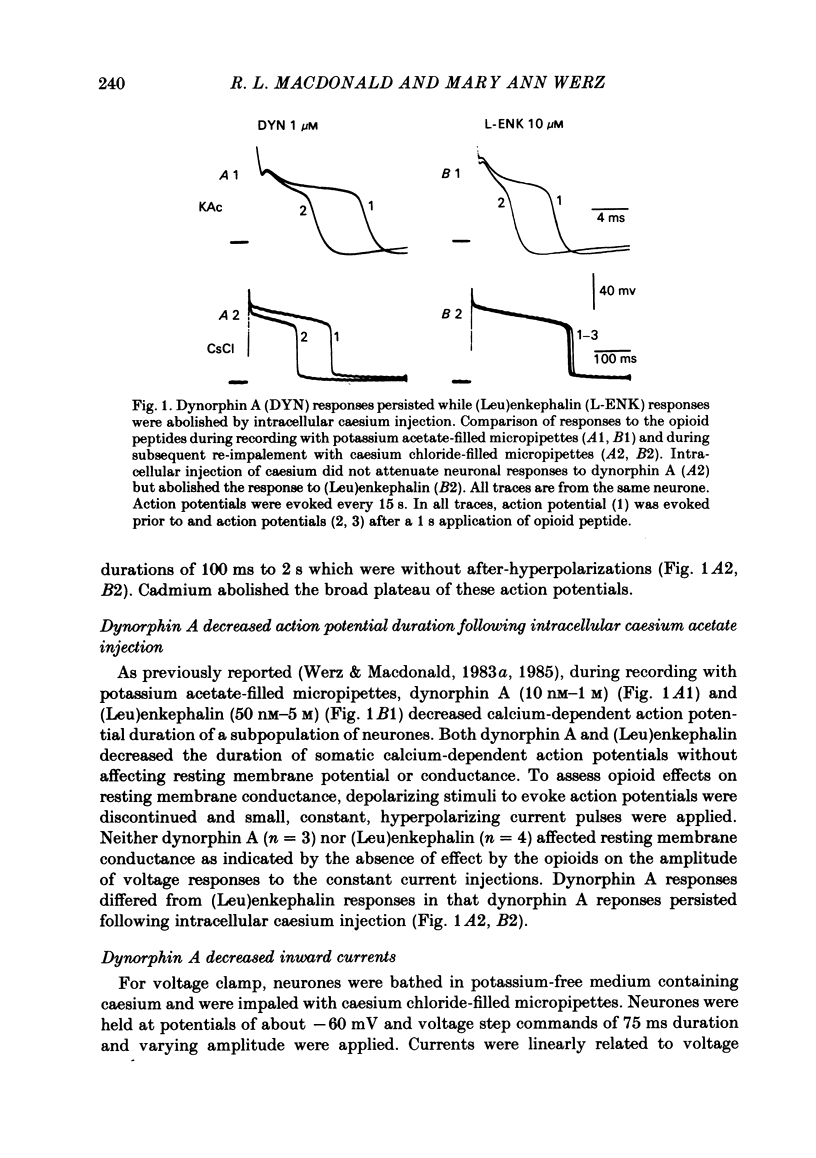
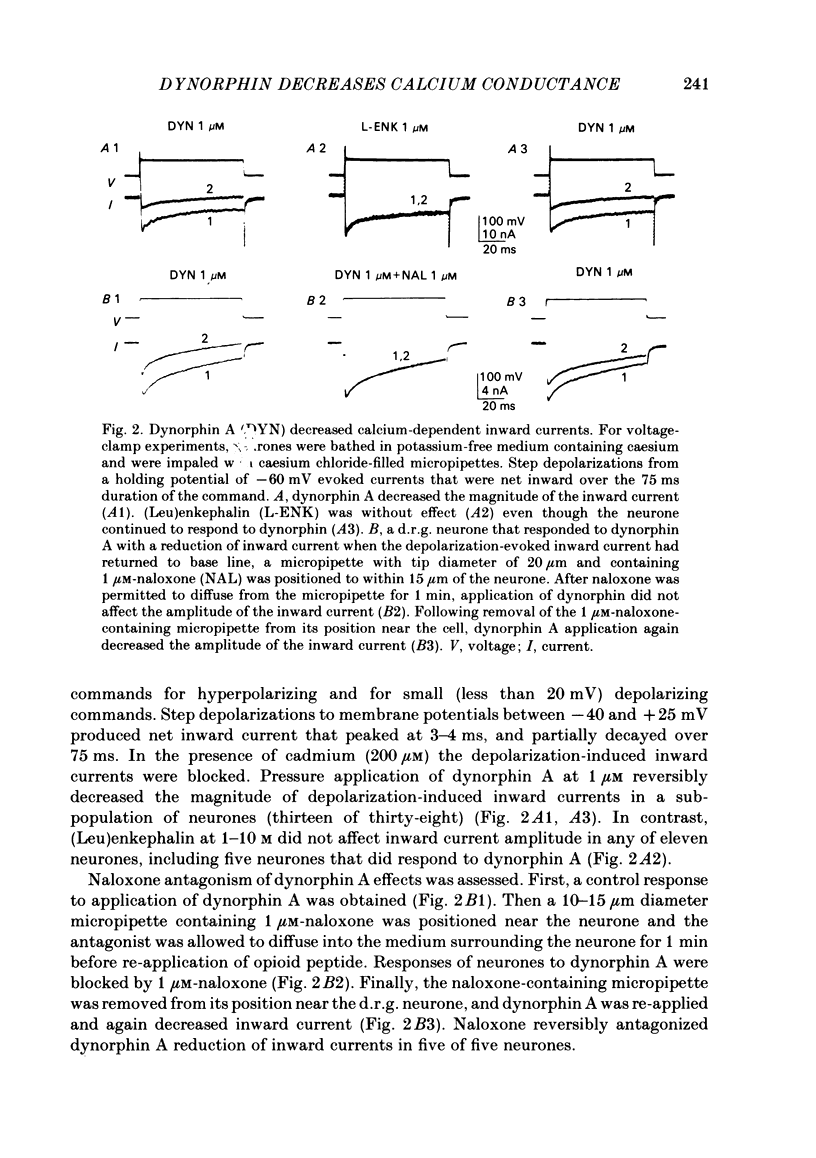
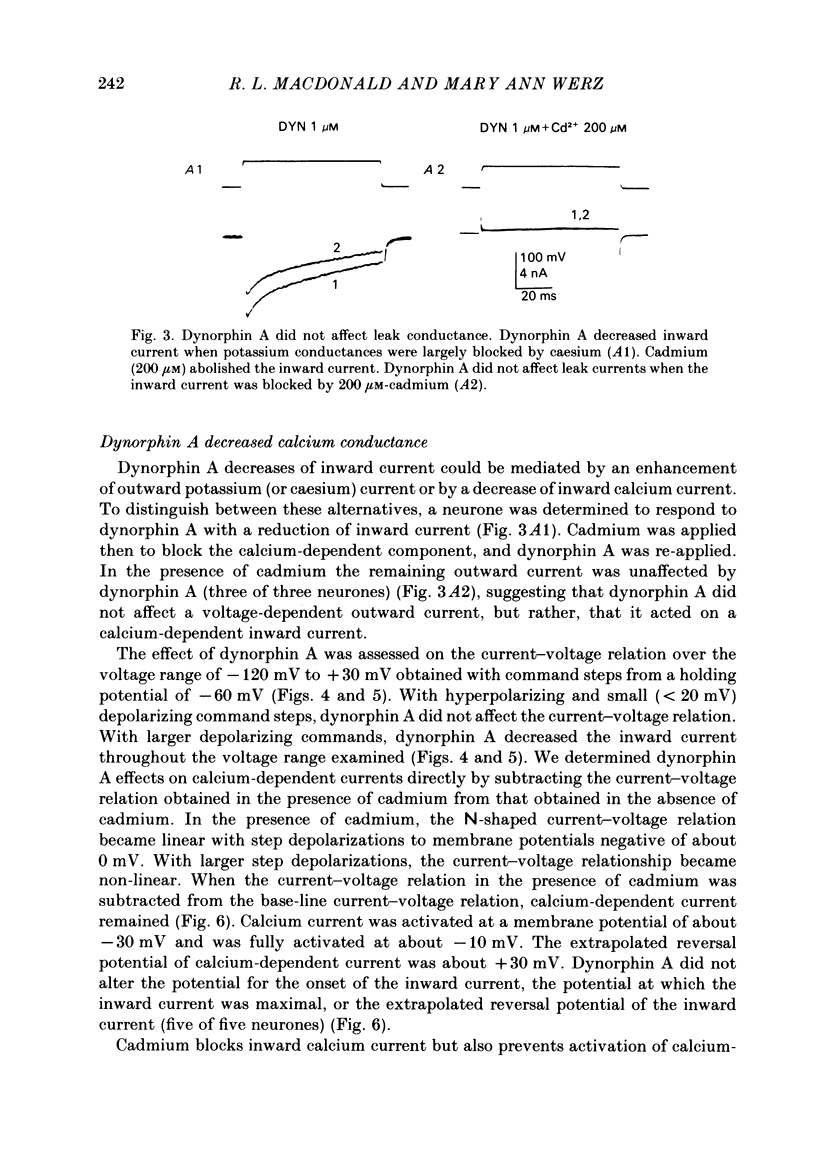
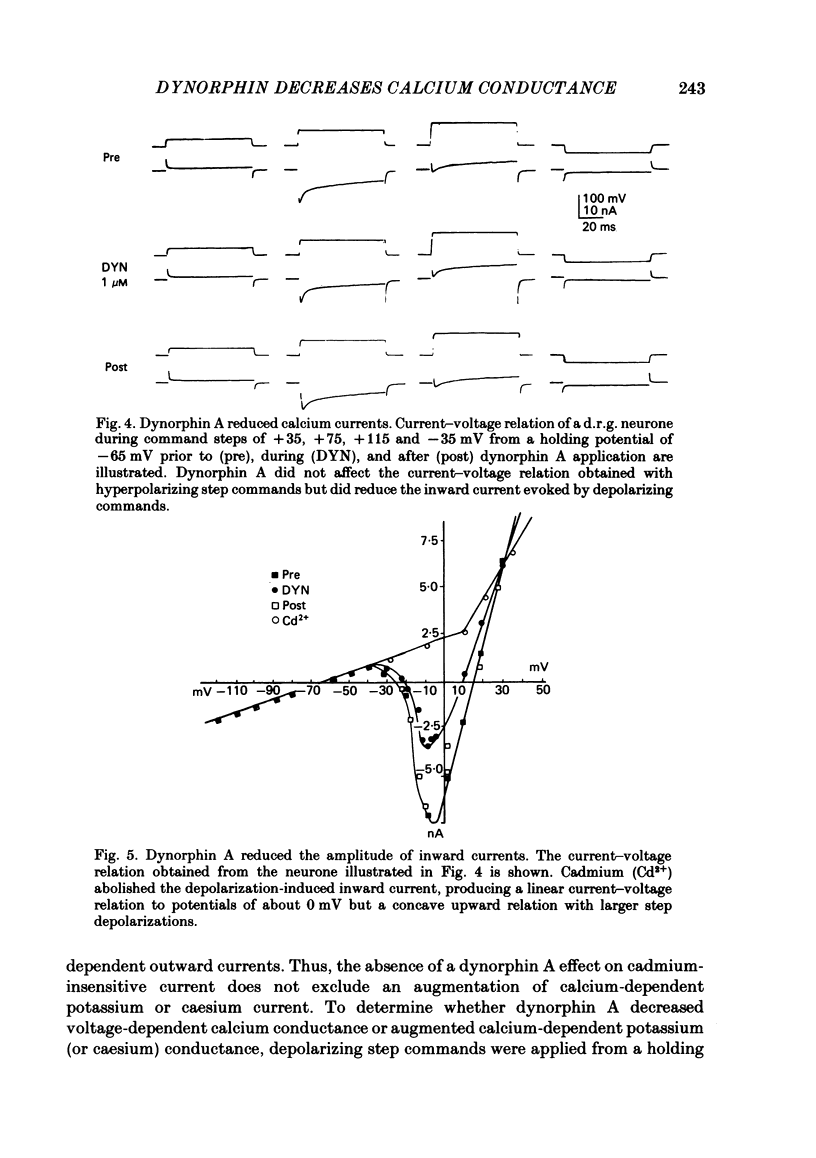
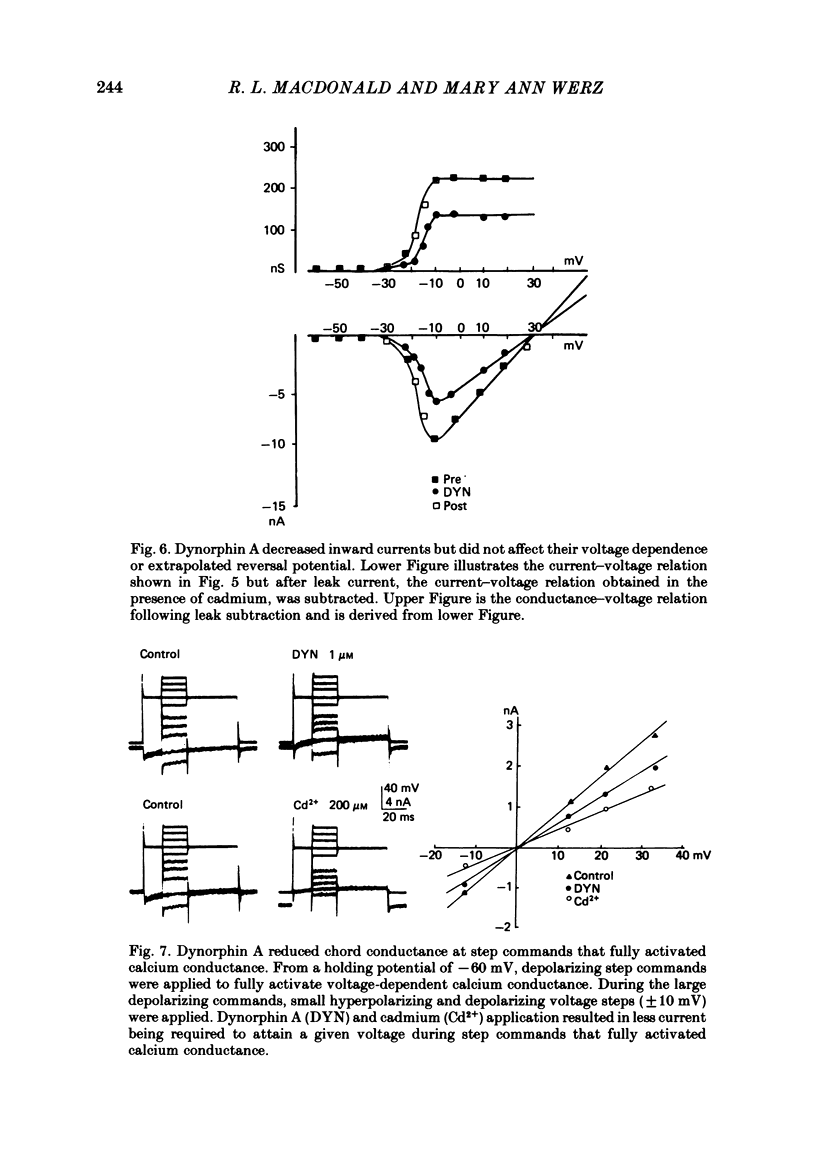
The actions of the opioid peptides dynorphin A and (Leu)enkephalin were assessed on calcium-dependent action potentials and inward calcium currents recorded from somata of mouse dorsal root ganglion (d.r.g.) neurones grown in primary dissociated cell culture. Dynorphin A and (Leu)enkephalin decreased the duration of somatic calcium-dependent action potentials in a portion of d.r.g. neurones impaled with potassium acetate-filled micropipettes. When substantial potassium conductance was blocked by intracellular injection of caesium acetate, d.r.g. neurones continued to respond to dynorphin A but responses to (Leu)enkephalin were abolished. In voltage-clamp experiments, dynorphin A but not (Leu)enkephalin reduced the magnitude of inward calcium currents. Dynorphin A responses were blocked by the opiate antagonist naloxone. The dynorphin A effect was due to reduction of voltage-dependent calcium conductance since dynorphin A reduced depolarization-evoked inward currents but did not alter membrane conductance following blockade of calcium channels by cadmium, and because dynorphin A reduced the instantaneous current-voltage slope (chord conductance) during step commands that produced maximal activation of voltage-dependent calcium conductance. Dynorphin A binds with high affinity to kappa-opioid receptors. (Leu)enkephalin, which has affinity for both mu- and delta-receptors but not for kappa-opioid receptors, was without effect on calcium conductance. Therefore, we suggest that kappa-receptors are coupled to voltage-dependent calcium-channels and that binding of dynorphin A produces a decrease of calcium current.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Cooper B. R., Hazum E., Cuatrecasas P. Multiple opiate receptors: different regional distribution in the brain and differential binding of opiates and opioid peptides. Mol Pharmacol. 1979 Jul;16(1):91–104. [PubMed] [Google Scholar]
- Chang K. J., Hazum E., Cuatrecasas P. Possible role of distinct morphine and enkephalin receptors in mediating actins of benzomorphan drugs (putative kappa and sigma agonists). Proc Natl Acad Sci U S A. 1980 Aug;77(8):4469–4473. doi: 10.1073/pnas.77.8.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature. 1981 Jun 18;291(5816):591–593. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Emson P. C., Leigh B. K., Gilbert R. F., Iversen L. L. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980 Mar 27;284(5754):351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H. Autoradiographic localization of kappa opiate receptors to deep layers of the cerebral cortex may explain unique sedative and analgesic effects. Life Sci. 1982 Sep 20;31(12-13):1291–1294. doi: 10.1016/0024-3205(82)90364-2. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Calcium- and sodium-dependent action potentials of mouse spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1982 Apr;47(4):641–655. doi: 10.1152/jn.1982.47.4.641. [DOI] [PubMed] [Google Scholar]
- Hiller J. M., Simon E. J., Crain S. M., Peterson E. R. Opiate receptors in cultures of fetal mouse dorsal root ganglia (DRG) and spinal cord: predominance in DRG neurites. Brain Res. 1978 Apr 28;145(2):396–400. doi: 10.1016/0006-8993(78)90875-2. [DOI] [PubMed] [Google Scholar]
- James I. F., Goldstein A. Site-directed alkylation of multiple opioid receptors. I. Binding selectivity. Mol Pharmacol. 1984 May;25(3):337–342. [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J., Robson L. E. Characterization of the kappa-subtype of the opiate receptor in the guinea-pig brain. Br J Pharmacol. 1981 Aug;73(4):939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., VonVoigtlander P. F., Barsuhn C. Properties of a selective kappa agonist, U-50,488H. Life Sci. 1982 Nov 15;31(20-21):2257–2260. doi: 10.1016/0024-3205(82)90132-1. [DOI] [PubMed] [Google Scholar]
- Lamotte C., Pert C. B., Snyder S. H. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976 Aug 13;112(2):407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Nelson P. G. Specific-opiate-induced depression of transmitter release from dorsal root ganglion cells in culture. Science. 1978 Mar 31;199(4336):1449–1451. doi: 10.1126/science.204015. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res. 1982 Jun 17;242(1):145–150. doi: 10.1016/0006-8993(82)90504-2. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic M., Hunt S. P., Gleave J. R. Localization of opiate and histamine H1-receptors in the primate sensory ganglia and spinal cord. Brain Res. 1982 Jun 10;241(2):197–206. doi: 10.1016/0006-8993(82)91056-3. [DOI] [PubMed] [Google Scholar]
- North R. A., Tonini M. The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br J Pharmacol. 1977 Dec;61(4):541–549. doi: 10.1111/j.1476-5381.1977.tb07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T. Opiate activation of potassium conductance inhibits calcium action potentials in rat locus coeruleus neurones. Br J Pharmacol. 1983 Oct;80(2):225–228. doi: 10.1111/j.1476-5381.1983.tb10023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C. M., Henderson G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980 Jul 18;209(4454):394–395. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Christian C. N., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. II. Synaptic activity and circuit behavior. J Neurophysiol. 1977 Sep;40(5):1151–1162. doi: 10.1152/jn.1977.40.5.1151. [DOI] [PubMed] [Google Scholar]
- Robson L. E., Kosterlitz H. W. Specific protection of the binding sites of D-Ala2-D-Leu5-enkephalin (delta-receptors) and dihydromorphine (mu-receptors). Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):425–432. doi: 10.1098/rspb.1979.0076. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T. L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):1–12. [PubMed] [Google Scholar]
- Tokimasa T., Morita K., North A. Opiates and clonidine prolong calcium-dependent after-hyperpolarizations. Nature. 1981 Nov 12;294(5837):162–163. doi: 10.1038/294162a0. [DOI] [PubMed] [Google Scholar]
- Upton N., Sewell R. D., Spencer P. S. Differentiation of potent mu and kappa-opiate agonists using heat and pressure antinociceptive profiles and combined potency analysis. Eur J Pharmacol. 1982 Mar 26;78(4):421–429. doi: 10.1016/0014-2999(82)90484-8. [DOI] [PubMed] [Google Scholar]
- Werz M. A., MacDonald R. L. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett. 1983 Dec 2;42(2):173–178. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Dynorphin and neoendorphin peptides decrease dorsal root ganglion neuron calcium-dependent action potential duration. J Pharmacol Exp Ther. 1985 Jul;234(1):49–56. [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Dynorphin reduces calcium-dependent action potential duration by decreasing voltage-dependent calcium conductance. Neurosci Lett. 1984 May 4;46(2):185–190. doi: 10.1016/0304-3940(84)90439-7. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Dynorphin reduces voltage-dependent calcium conductance of mouse dorsal root ganglion neurons. Neuropeptides. 1984 Dec;5(1-3):253–256. doi: 10.1016/0143-4179(84)90075-1. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu- and delta-opiate receptors. Nature. 1982 Oct 21;299(5885):730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Opioid peptides decrease calcium-dependent action potential duration of mouse dorsal root ganglion neurons in cell culture. Brain Res. 1982 May 6;239(1):315–321. doi: 10.1016/0006-8993(82)90859-9. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent action potentials. J Pharmacol Exp Ther. 1983 Nov;227(2):394–402. [PubMed] [Google Scholar]
- Williams J. T., Egan T. M., North R. A. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982 Sep 2;299(5878):74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Wood P. L., Rackham A., Richard J. Spinal analgesia: comparison of the mu agonist morphine and the kappa agonist ethylketazocine. Life Sci. 1981 May 11;28(19):2119–2125. doi: 10.1016/0024-3205(81)90618-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]


