Abstract
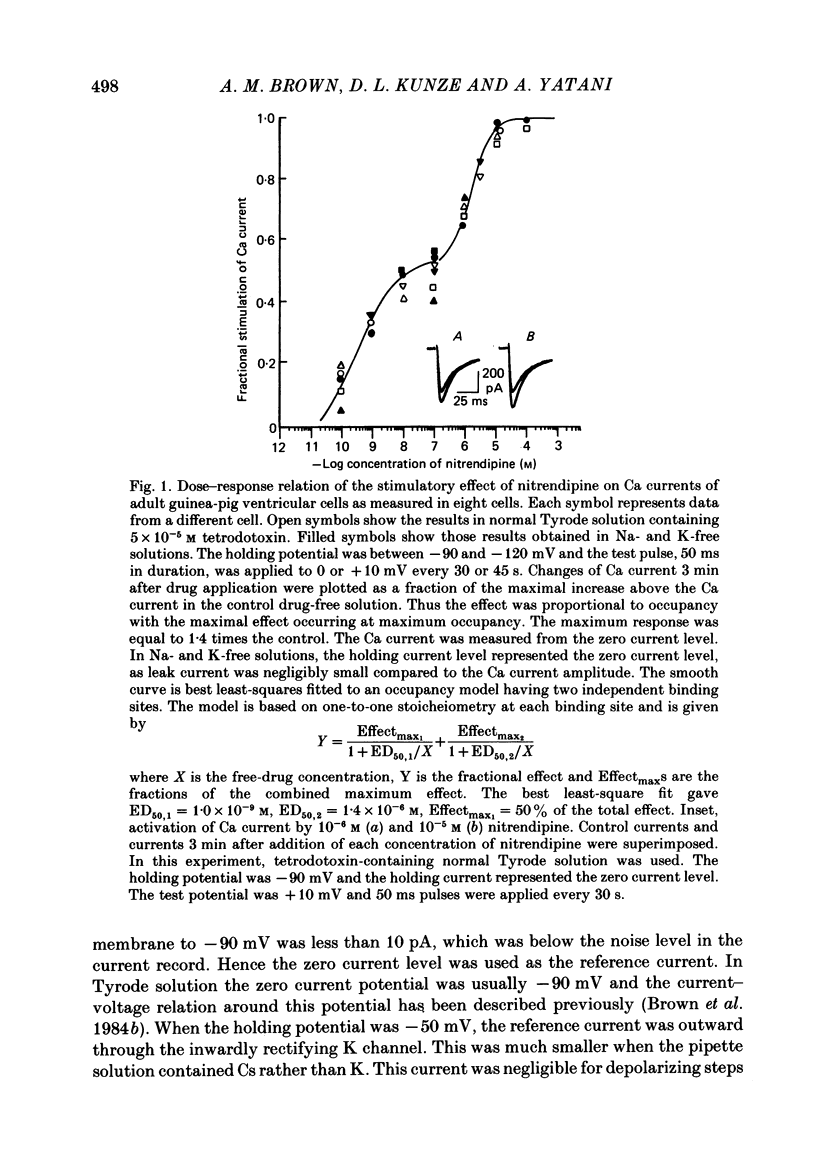
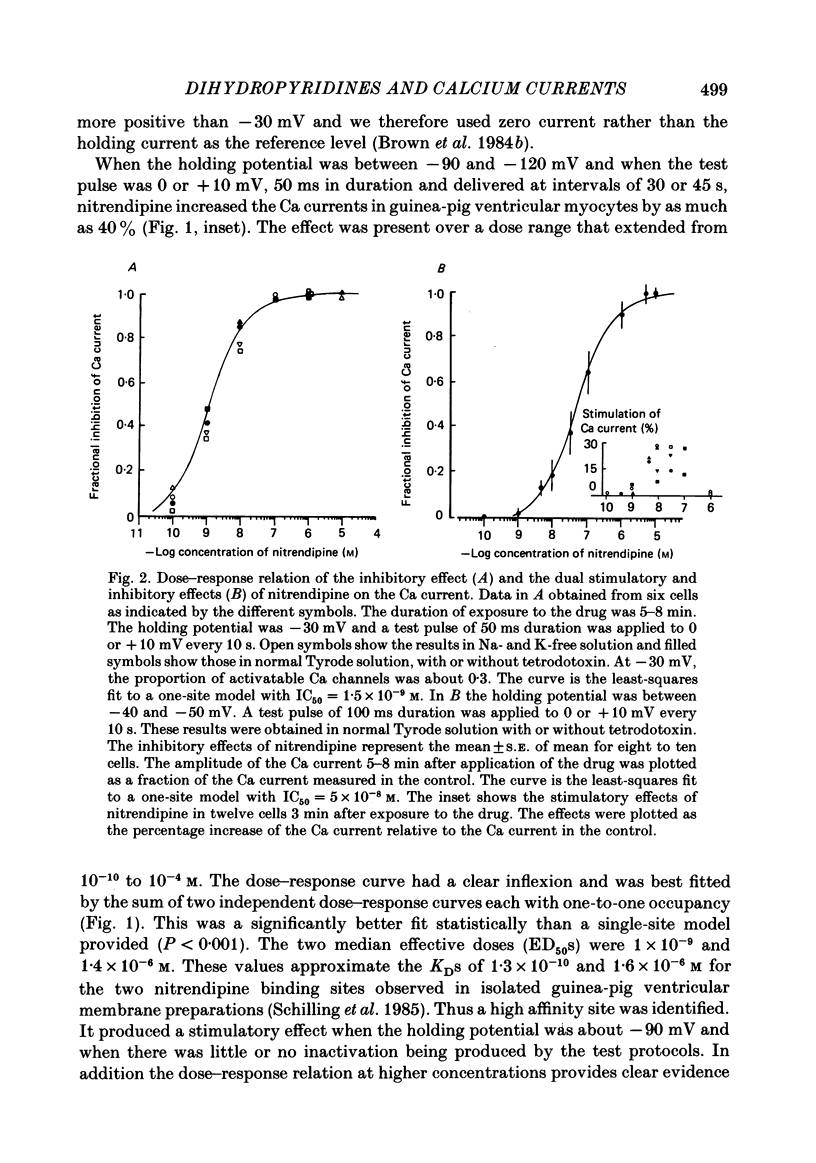
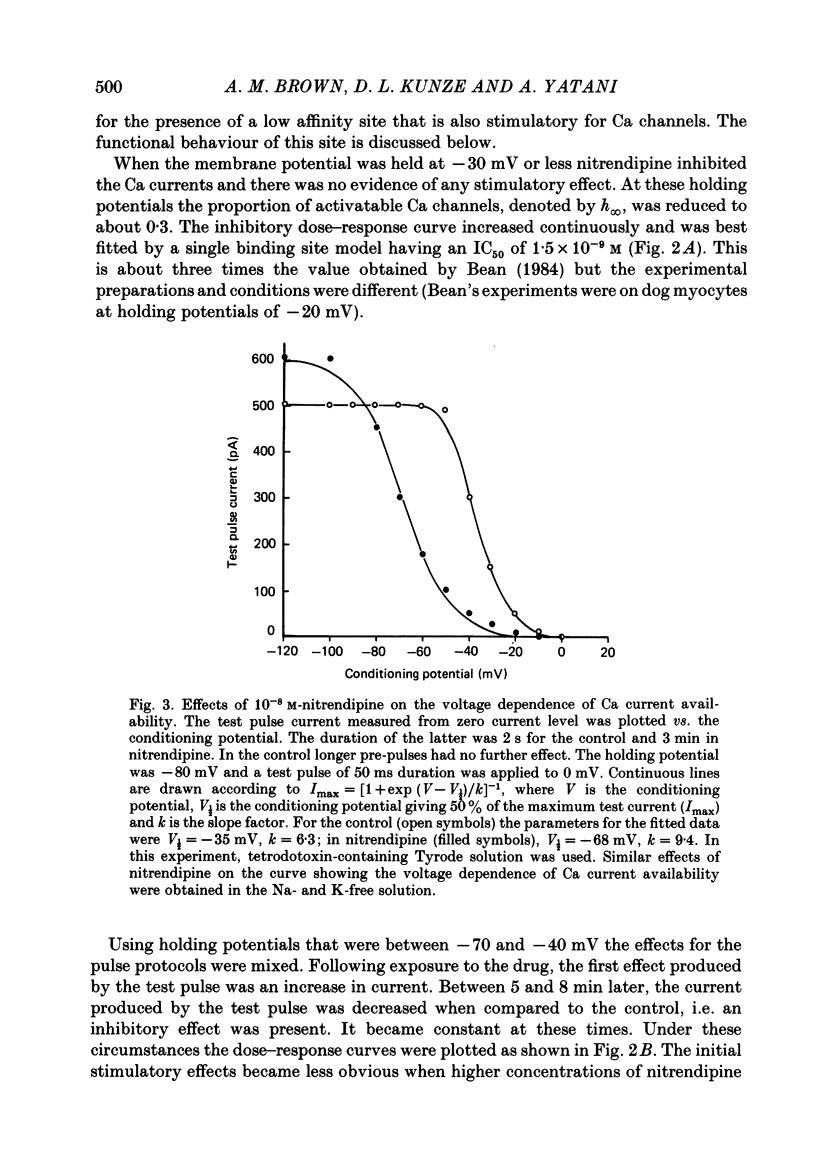
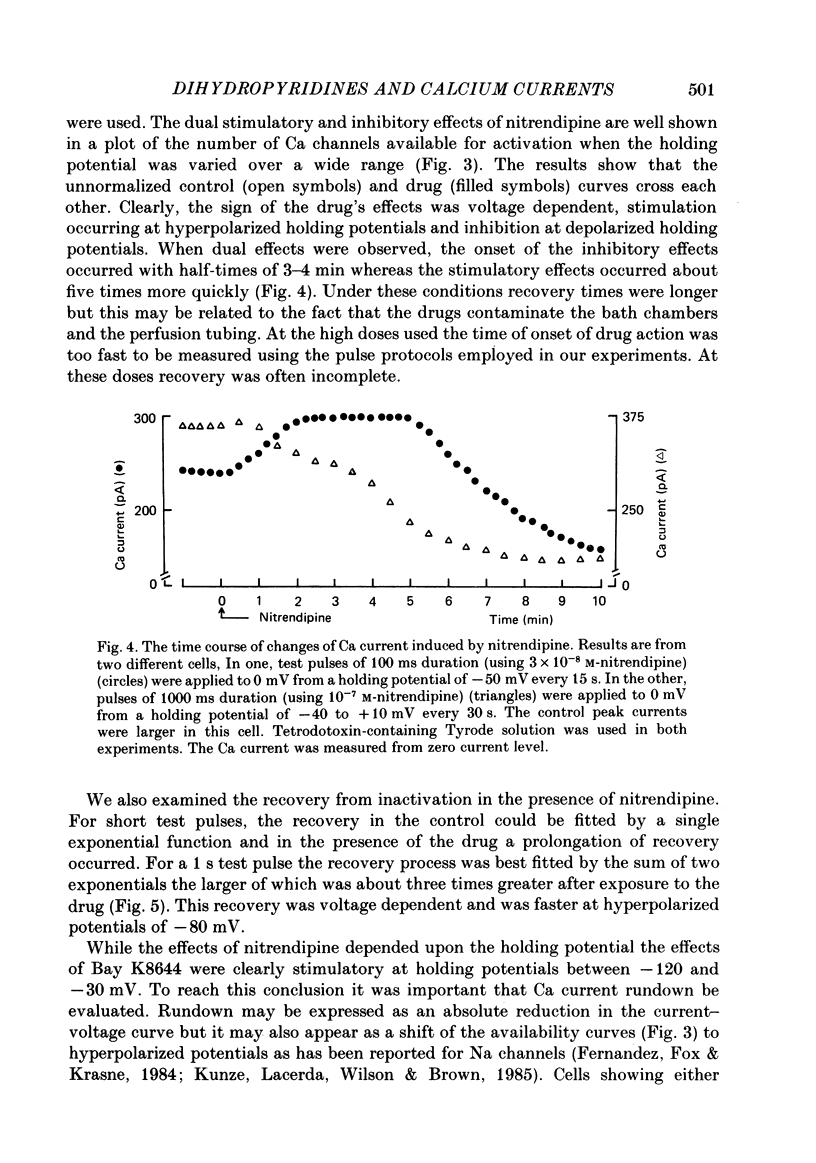
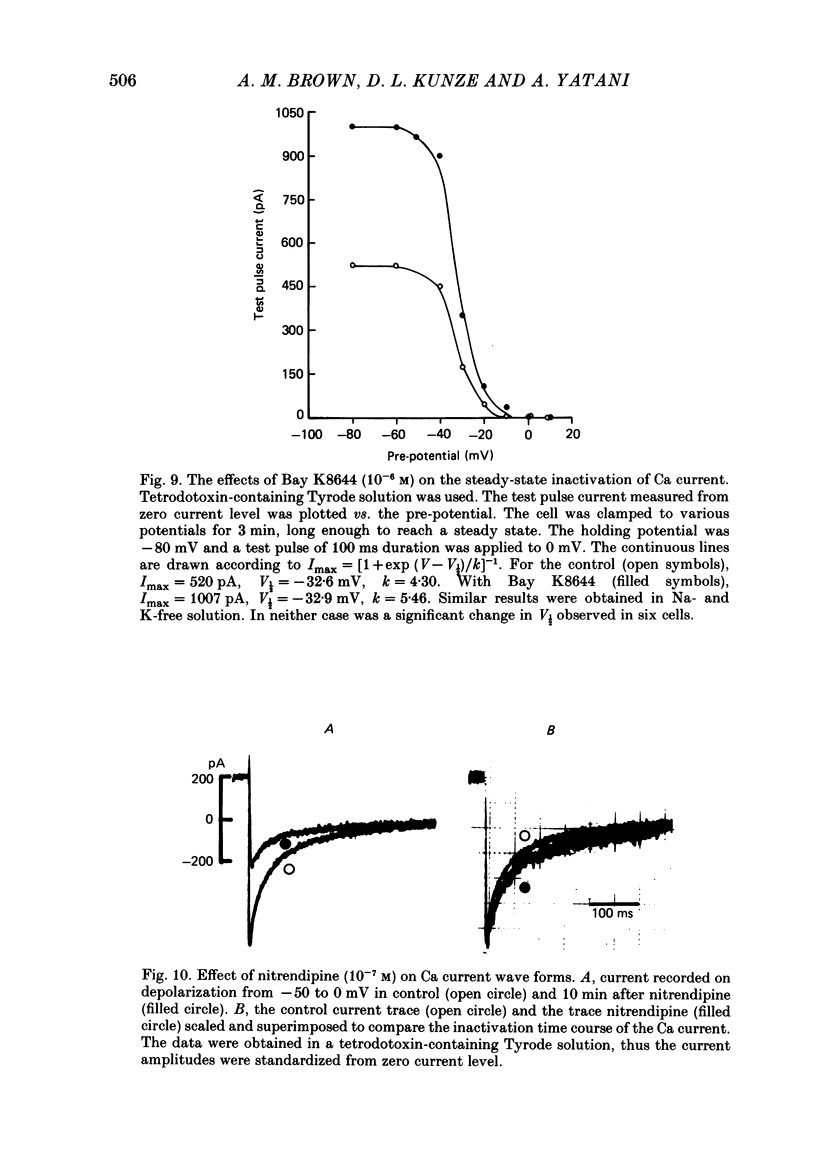
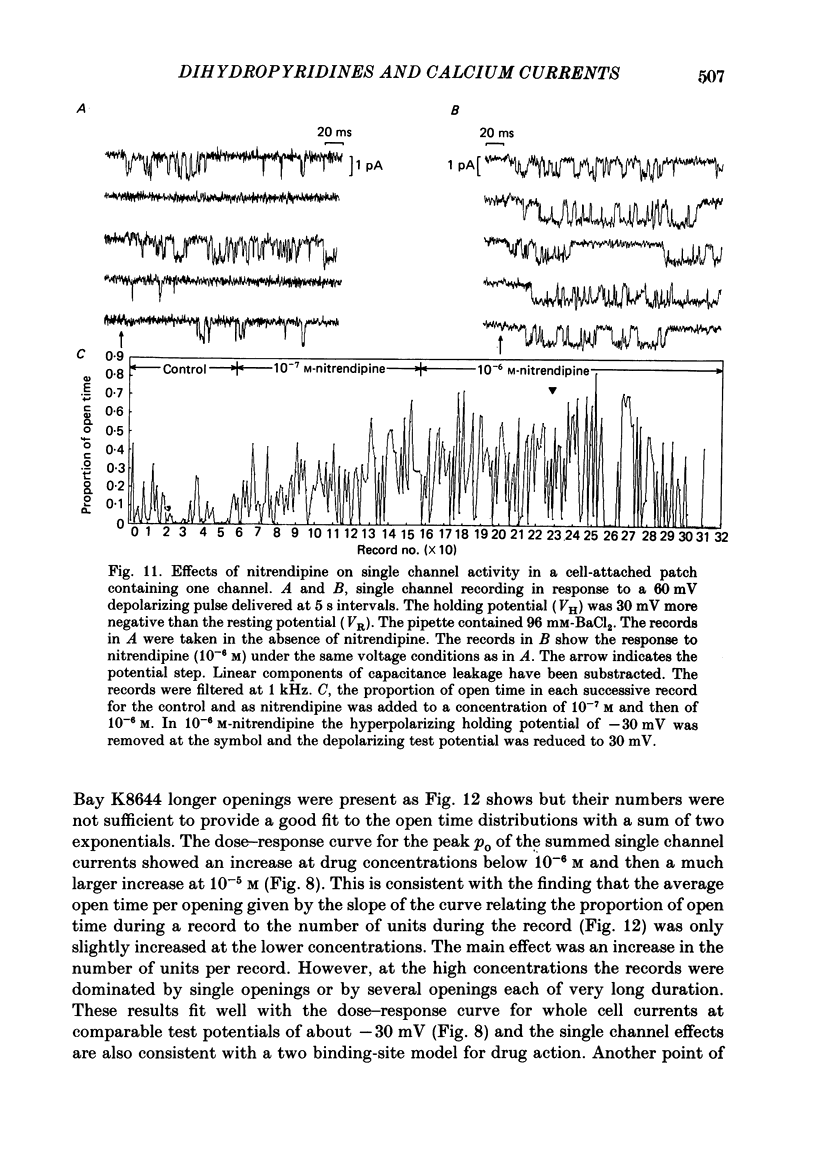
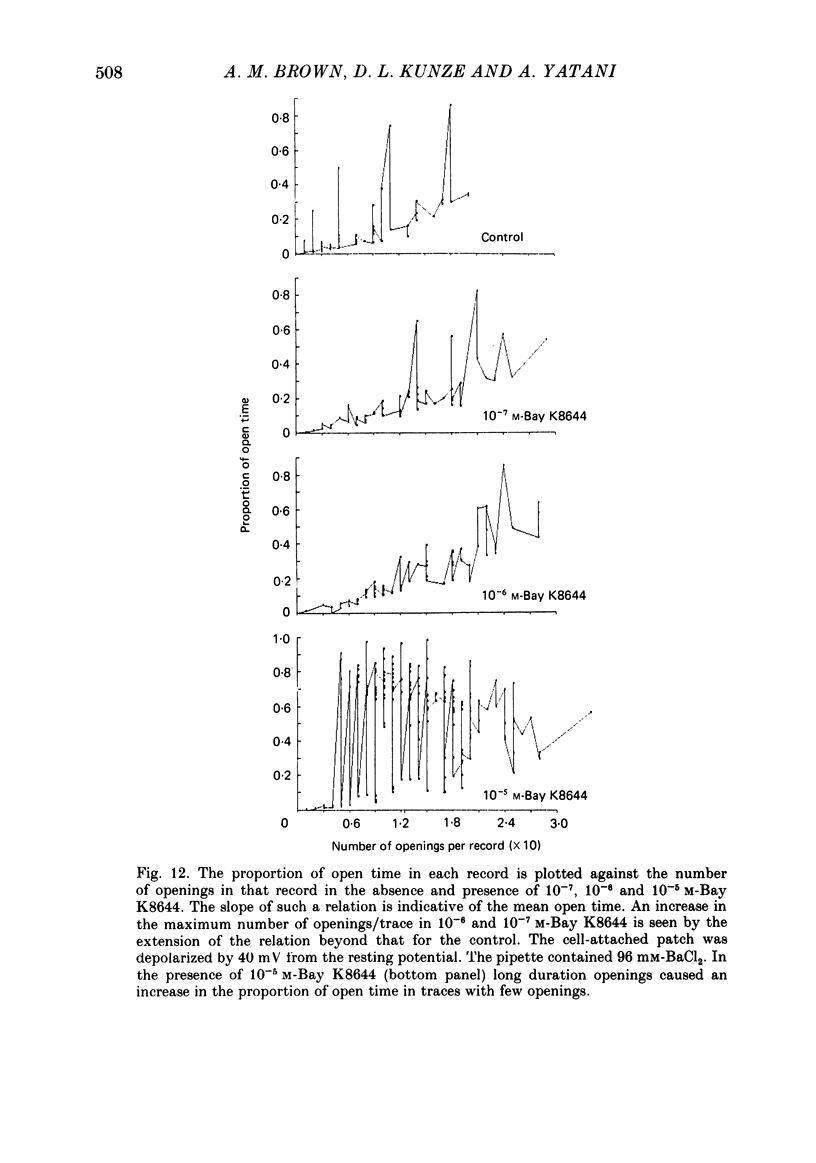
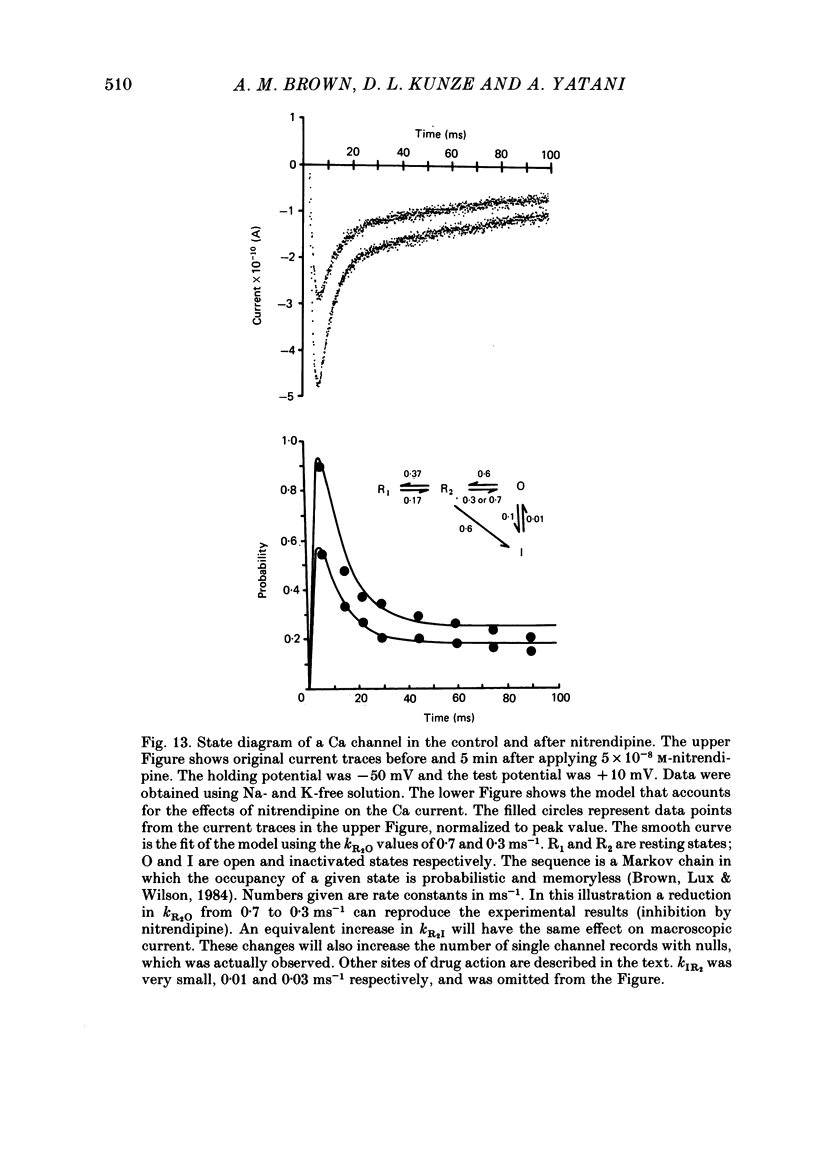
We studied the effects of dihydropyridine Ca channel ligands (DHPs), mainly nitrendipine and Bay K8644, on whole cell and single channel Ca currents on single myocytes isolated from the adult guinea-pig ventricle. Nitrendipine had dual effects, stimulatory or inhibitory, depending upon the membrane potential. At low frequencies (less than 0.03 Hz) and negative holding potentials (-90 mV or more), nitrendipine increased the Ca currents in a dose-dependent manner. The dose-response curve was best fitted by a Langmuir adsorption isotherm model which was the sum of two independent one-to-one drug-receptor sites with median effective doses (ED50S) of 1.0 X 10(-9) M and 1.4 X 10(-6) M respectively. When the membrane potential was held at -30 mV or less, nitrendipine inhibited the Ca currents, also in a dose-dependent manner. The dose-response curve was fitted by a single binding site model having a median inhibitor concentration (IC50) of 1.5 X 10(-9) M. At holding potentials between -70 and -40 mV, nitrendipine produced mixed effects on Ca currents; an increase occurred initially and this was followed by a decrease. When rundown was excluded, Bay K8644 showed only stimulatory effects on the Ca currents between holding potentials of -120 and -30 mV. When the test potential was zero or +10 mV the Ca currents reached peak values and the dose-response curve was best fitted by a single binding site model having an ED50 of 3 X 10(-8) M. When the effects were measured at negative test potentials of -30 to -10 mV, the curve was best fitted by a two-site model with ED50S of 3 X 10(-9) and 9 X 10(-7) M. At the single Ca channel level the stimulatory effect of nitrendipine was due to an increased probability that a Ca channel which had opened once would reopen, a reduction in records without activity and an increase in the mean open time. There were no changes in unit conductance. Inhibitory effects were due to a large increase in nulls. At lower concentrations the main effect of Bay K8644 was an increase in the probability of opening. At doses above 10(-6) M, a pronounced increase in the open time was observed. The effects we observed are attributed to at least two sites for DHP related to Ca channels; one with high affinity and one with a lower affinity. The low affinity site mediates a stimulatory effect due to greatly prolonged openings.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemann P., Ferry D., Lübbecke F., Glossman H. [3H]-Nitrendipine, a potent calcium antagonist, binds with high affinity to cardiac membranes. Arzneimittelforschung. 1981;31(12):2064–2067. [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lux H. D., Wilson D. L. Activation and inactivation of single calcium channels in snail neurons. J Gen Physiol. 1984 May;83(5):751–769. doi: 10.1085/jgp.83.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H. Activation of chromaffin cell Ca2+ channels by novel dihydropyridine. Nature. 1985 Feb 7;313(6002):503–504. doi: 10.1038/313503c0. [DOI] [PubMed] [Google Scholar]
- Gould R. J., Murphy K. M., Snyder S. H. [3H]nitrendipine-labeled calcium channels discriminate inorganic calcium agonists and antagonists. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3656–3660. doi: 10.1073/pnas.79.11.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., Yatani A., Hawkes M. J., Redding K., Brown A. M. Atrotoxin: a specific agonist for calcium currents in heart. Science. 1985 Jul 12;229(4709):182–184. doi: 10.1126/science.3160111. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hof R. P., Rüegg U. T., Hof A., Vogel A. Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol. 1985 Jul-Aug;7(4):689–693. doi: 10.1097/00005344-198507000-00012. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hume J. R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J Pharmacol Exp Ther. 1985 Jul;234(1):134–140. [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Patch and whole cell calcium currents recorded simultaneously in snail neurons. J Gen Physiol. 1984 May;83(5):727–750. doi: 10.1085/jgp.83.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. D., Loh E., Lachance D., Barry W. H., Smith T. W. Relationship of binding of a calcium channel blocker to inhibition of contraction in intact cultured embryonic chick ventricular cells. Circ Res. 1983 Oct;53(4):539–543. doi: 10.1161/01.res.53.4.539. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Regulation of cardiac calcium channel current and contractile activity by the dihydropyridine Bay K 8644 is voltage-dependent. J Mol Cell Cardiol. 1984 Jul;16(7):667–670. doi: 10.1016/s0022-2828(84)80631-8. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Grupp I. L., Grupp G., Williams J. S., Vaghy P. L. Effects of dihydropyridine calcium channel modulators in the heart: pharmacological and radioligand binding correlations. Biochem Biophys Res Commun. 1984 Nov 30;125(1):387–394. doi: 10.1016/s0006-291x(84)80380-0. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Triggle D. J. Cellular action of calcium channel blocking drugs. Annu Rev Med. 1984;35:325–339. doi: 10.1146/annurev.me.35.020184.001545. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O., Strauss H. C. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984 Jul;46(1):15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Gross R., Schramm M. Calcium channel modulation: ability to inhibit or promote calcium influx resides in the same dihydropyridine molecule. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1170–1176. [PubMed] [Google Scholar]
- Vaghy P. L., Grupp I. L., Grupp G., Balwierczak J. L., Williams J. S., Schwartz A. Correlation of nitrendipine and Bay k 8644 binding to isolated canine heart sarcolemma with their pharmacological effects on the canine heart. Eur J Pharmacol. 1984 Jul 13;102(2):373–374. doi: 10.1016/0014-2999(84)90273-5. [DOI] [PubMed] [Google Scholar]
- Williams J. S., Grupp I. L., Grupp G., Vaghy P. L., Dumont L., Schwartz A., Yatani A., Hamilton S., Brown A. M. Profile of the oppositely acting enantiomers of the dihydropyridine 202-791 in cardiac preparations: receptor binding, electrophysiological, and pharmacological studies. Biochem Biophys Res Commun. 1985 Aug 30;131(1):13–21. doi: 10.1016/0006-291x(85)91763-2. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ Res. 1985 Jun;56(6):868–875. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]


