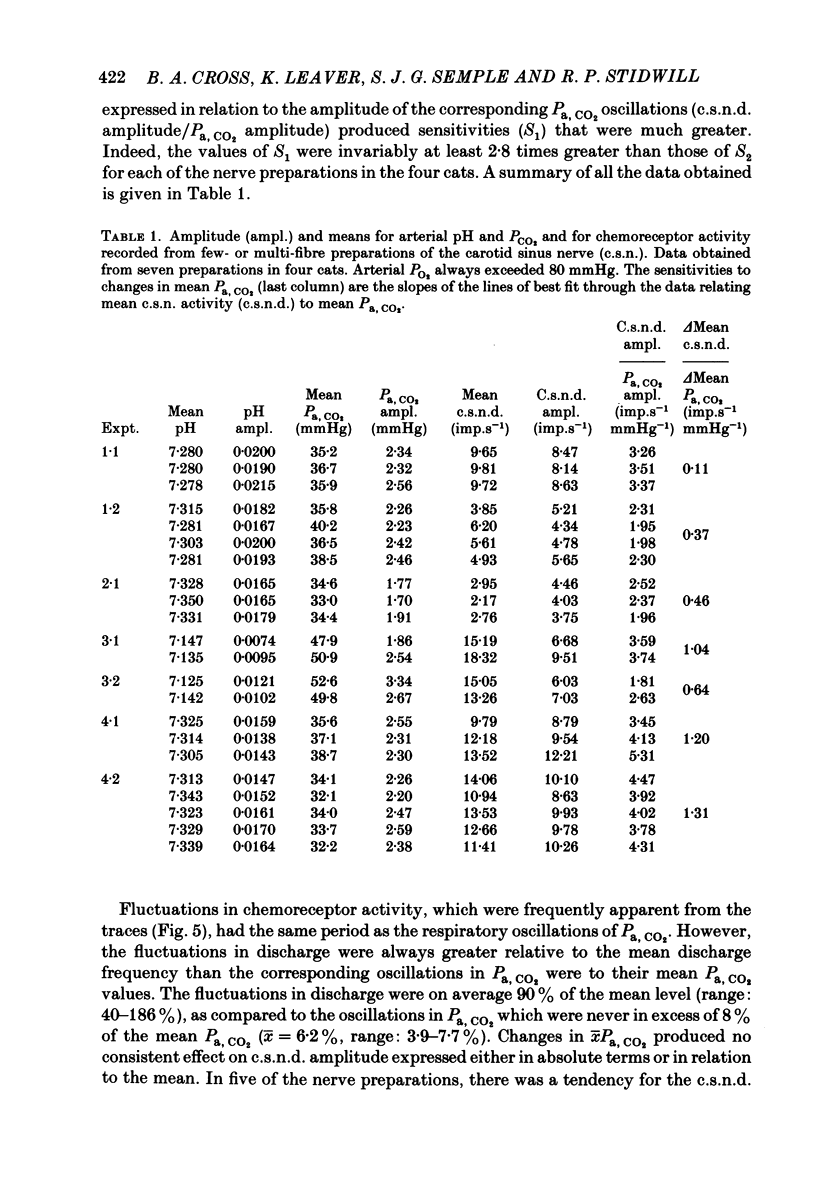
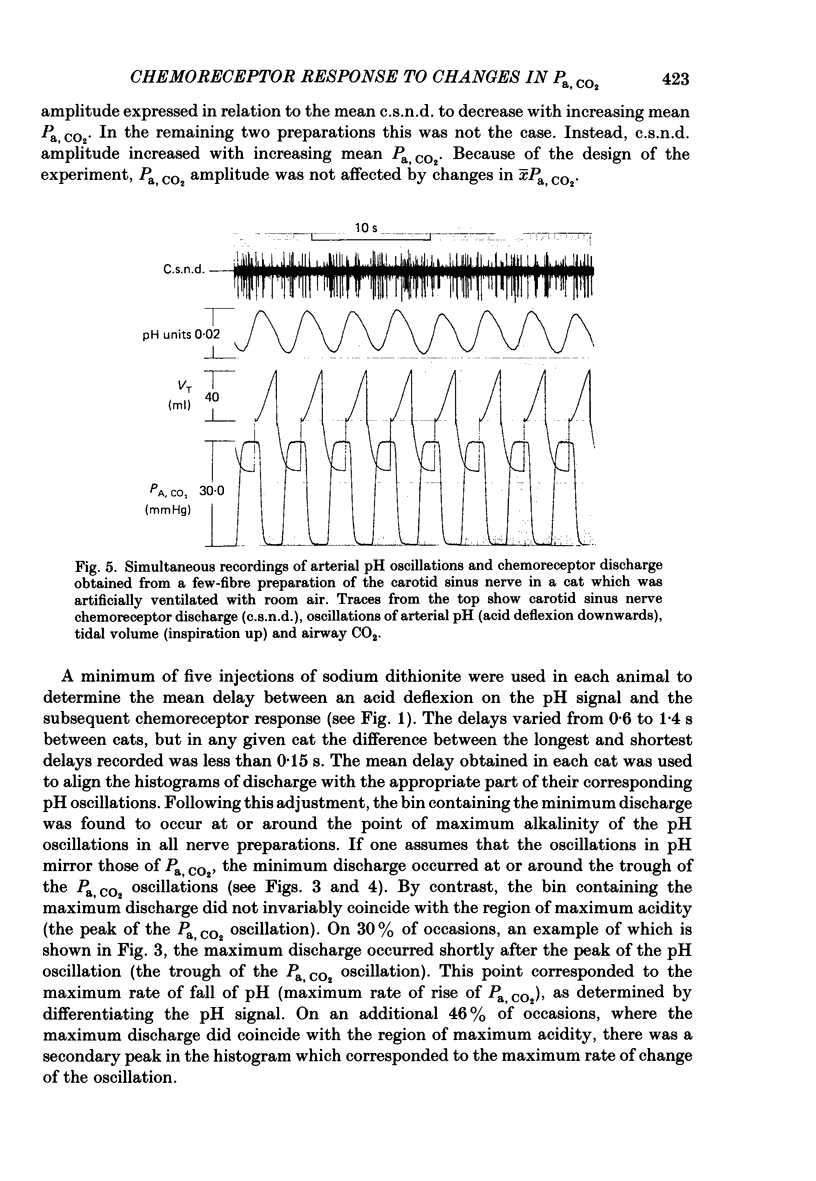
Abstract
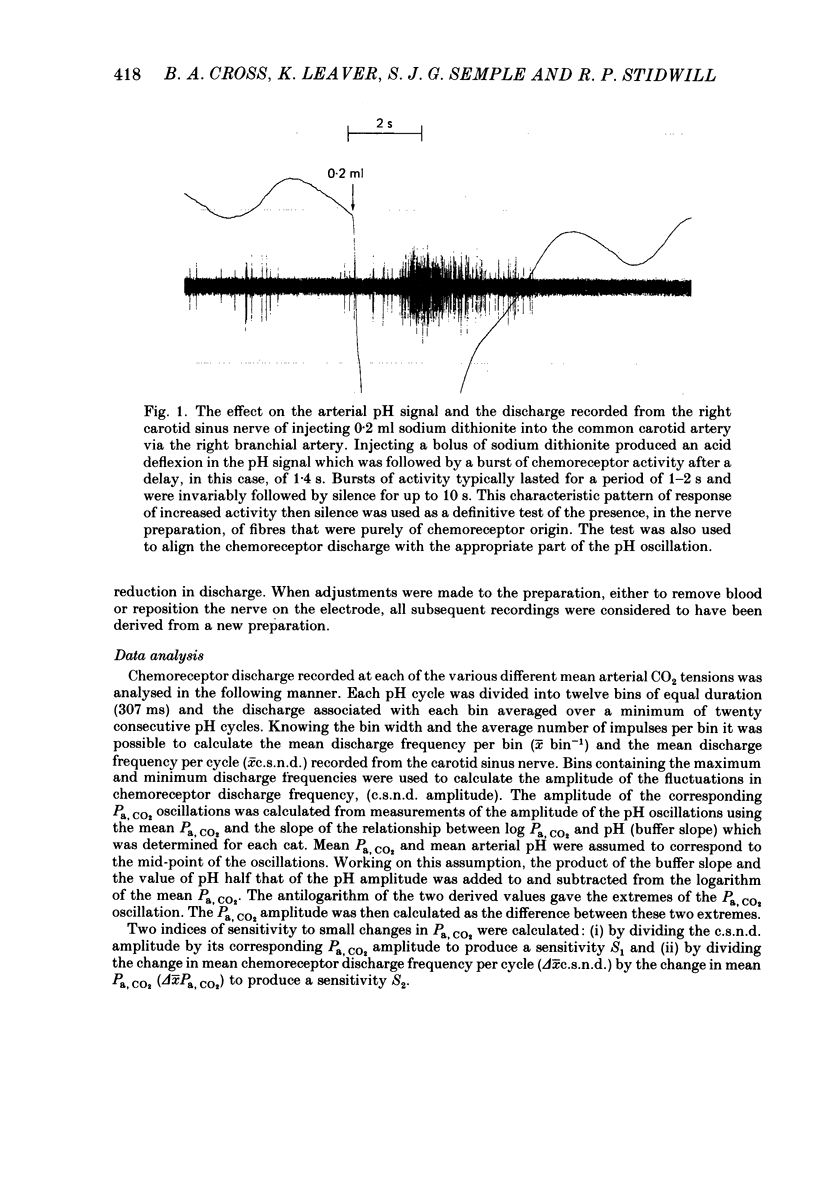
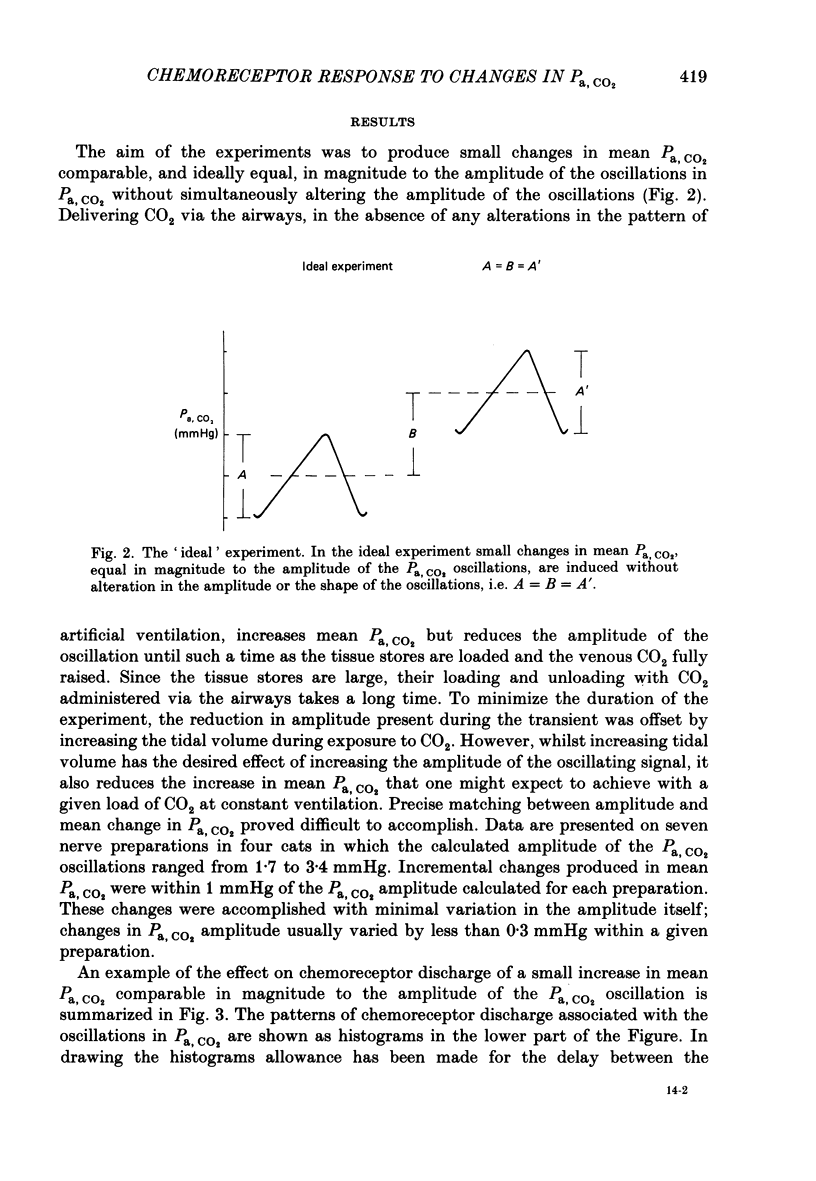
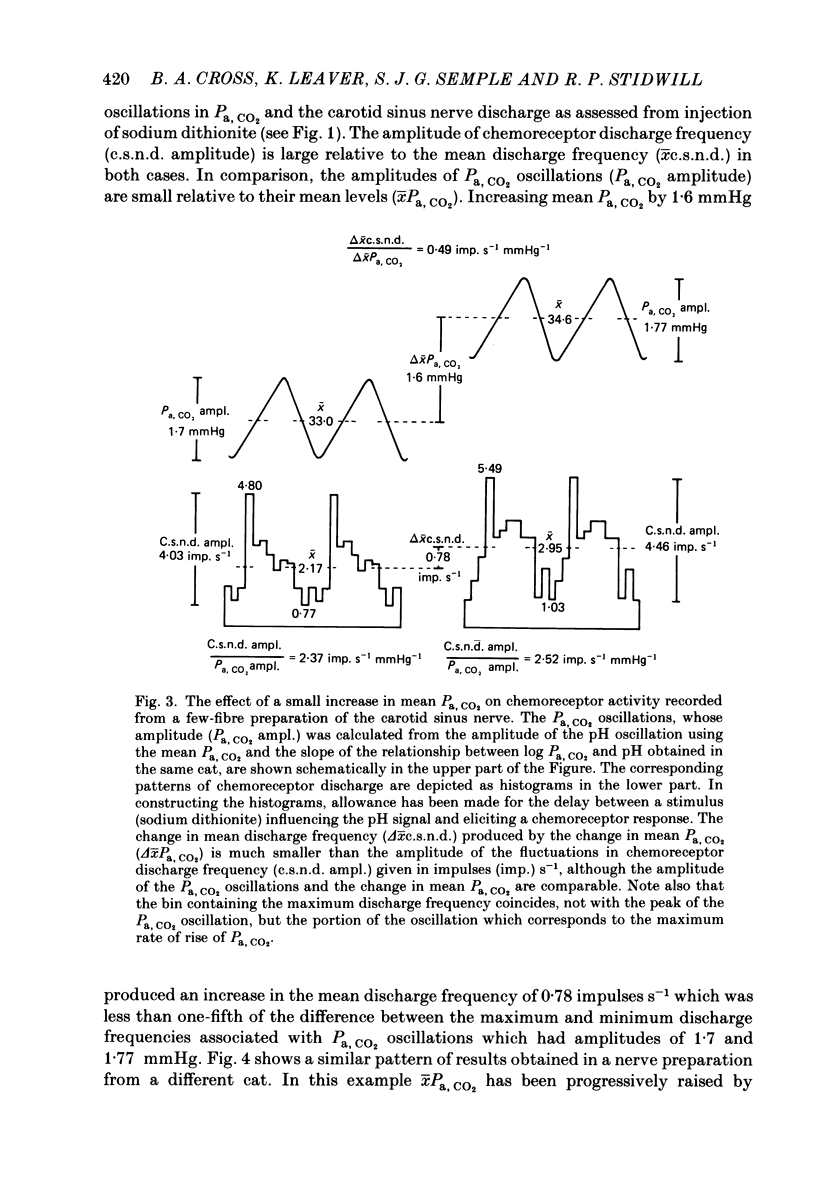
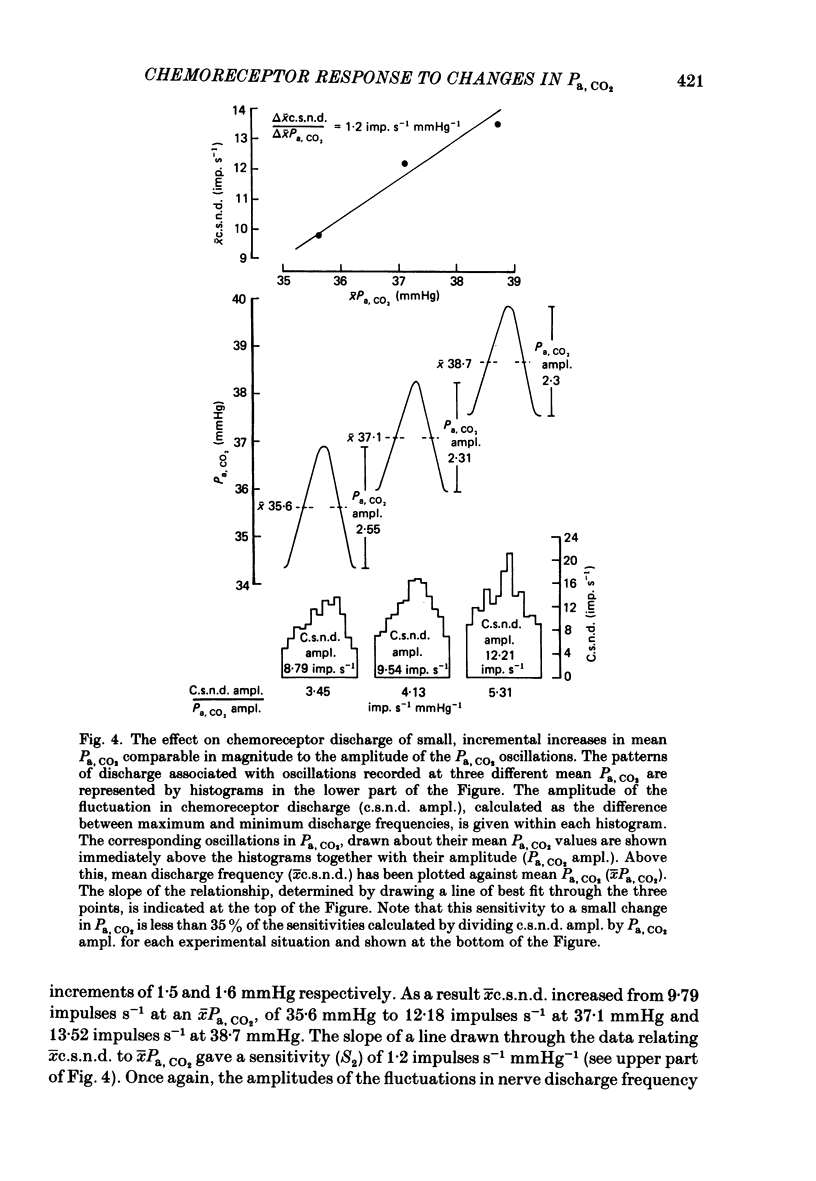
The hypothesis that the fluctuations in carotid chemoreceptor discharge represent more than a simple proportional response to respiratory oscillations of Pa, CO2 has been examined. Simultaneous recordings of the activity in few- or multi-fibre chemoreceptor preparations of the cut carotid sinus nerve and oscillations of carotid arterial pH have been made in four anaesthetized cats which were paralysed and artificially ventilated at constant frequency. Chemoreceptor activity was averaged over a minimum of twenty consecutive pH cycles and the amplitude of the fluctuations in discharge frequency determined. Using the mean Pa, CO2 obtained from blood-gas analysis and the slope of the relationship between log Pa, CO2 and pH obtained in each cat, the amplitude of the pH oscillations was converted to Pa, CO2 amplitude. This amplitude relative to the mean Pa, CO2 has been compared with the amplitude of the corresponding fluctuation in the chemoreceptor discharge frequency recorded from the carotid sinus nerve (e.s.n.d. amplitude) relative to its mean level. Whereas the Pa, CO2 amplitude was always less than 8% of the mean Pa, CO2, the c.s.n.d. amplitude ranged from 40 to 186% of the mean discharge frequency. C.s.n.d. amplitude was divided by the corresponding Pa, CO2 amplitude to give an index of the sensitivity of the chemoreceptors to Pa, CO2. This sensitivity has been compared with that determined from the mean discharge frequency produced in response to changes in mean Pa, CO2 comparable in magnitude to the Pa, CO2 amplitude. The former sensitivity was usually at least 3 times greater than the latter. Examination of the fluctuations in chemoreceptor discharge frequency in relation to the corresponding pH oscillations revealed that whereas the minimum discharge frequency coincided with the alkaline peak of the pH oscillation (trough of the Pa, CO2 oscillation), the maximum discharge frequency did not invariably coincide with the acid trough of the pH oscillation (peak of the Pa, CO2 oscillation). On 30% of occasions, the maximum discharge frequency was associated with the region of maximum rate of fall of pH (maximum rate of rise of Pa, CO2). It was concluded that fluctuations in the discharge of carotid chemoreceptors cannot be accounted for on the basis of a simple proportional relationship to Pa, CO2. They contain a large rate of change component.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band D. M., McClelland M., Phillips D. L., Saunders K. B., Wolff C. B. Sensitivity of the carotid body to within-breath changes in arterial PCO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):768–777. doi: 10.1152/jappl.1978.45.5.768. [DOI] [PubMed] [Google Scholar]
- Band D. M., Semple S. J. Continuous measurement of blood pH with an indwelling arterial glass electrode. J Appl Physiol. 1967 Apr;22(4):854–857. doi: 10.1152/jappl.1967.22.4.854. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., McCloskey D. I., Torrance R. W. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol. 1971 Oct;13(1):36–49. doi: 10.1016/0034-5687(71)90063-6. [DOI] [PubMed] [Google Scholar]
- Bsnd D. M., Saunders K. B., Wolff C. B. The relation between chemoreceptor discharge and respiratory fluctuation of arterial pH in the anaesthetized cat. J Physiol. 1971 Oct;218 (Suppl):73P–74P. [PubMed] [Google Scholar]
- Cowell T. K., Band D. M., Semple S. J. A fast-response pH meter. J Appl Physiol. 1967 Apr;22(4):858–860. doi: 10.1152/jappl.1967.22.4.858. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Leitner L. M., Liaubet M. J. Carotid chemoreceptor response to intermittent or sustained stimulation in the cat. Respir Physiol. 1969 Apr;6(3):395–402. doi: 10.1016/0034-5687(69)90037-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Parks D. C. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respir Physiol. 1971 Jun;12(2):218–229. doi: 10.1016/0034-5687(71)90054-5. [DOI] [PubMed] [Google Scholar]
- Gehrich J. L., Moore G. P. Statistical analysis of cyclic variations in carotid body chemoreceptor activity. J Appl Physiol. 1973 Nov;35(5):642–648. doi: 10.1152/jappl.1973.35.5.642. [DOI] [PubMed] [Google Scholar]
- Goodman N. W., Nail B. S., Torrance R. W. Oscillations in the discharge of single carotid chemorecptor fibers of the cat. Respir Physiol. 1974 Jun;20(3):251–269. doi: 10.1016/0034-5687(74)90023-1. [DOI] [PubMed] [Google Scholar]
- Lahiri S., DeLaney R. G. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975 Sep;24(3):249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]


