Abstract
With the aim of developing new functional foods, a traditional product, the table olive, was used as a vehicle for incorporating probiotic bacterial species. Survival on table olives of Lactobacillus rhamnosus (three strains), Lactobacillus paracasei (two strains), Bifidobacterium bifidum (one strain), and Bifidobacterium longum (one strain) at room temperature was investigated. The results obtained using a selected olive sample demonstrated that bifidobacteria and one strain of L. rhamnosus (Lactobacillus GG) showed a good survival rate, with a recovery of about 106 CFU g−1 after 30 days. The Lactobacillus GG population remained unvaried until the end of the experiment, while a slight decline (to about 105 CFU g−1) was observed for bifidobacteria. High viability, with more than 107 CFU g−1, was observed throughout the 3-month experiment for L. paracasei IMPC2.1. This strain, selected for its potential probiotic characteristics and for its lengthy survival on olives, was used to validate table olives as a carrier for transporting bacterial cells into the human gastrointestinal tract. L. paracasei IMPC2.1 was recovered from fecal samples in four out of five volunteers fed 10 to 15 olives per day carrying about 109 to 1010 viable cells for 10 days.
Over recent decades, the development and consumption of functional probiotic foods has been increasing alongside awareness of their beneficial effects in promoting gut health as well as in disease prevention and therapy, and this has raised interest in health-promoting foods (37). The contribution of probiotic bacteria, mainly lactobacilli and bifidobacteria, to maintaining or improving microbial balance in the gut is reviewed by Saarela et al. and by Salminen et al. (37, 38), and investigations are currently under way into their role in reducing the risk of cancer (11), influencing immunomodulatory features and preventing food allergies (22, 23, 38), counteracting hypercholesterolemia (37), and alleviating the symptoms of lactose intolerance (38).
The benefits derived from a regular intake of probiotic foods are also correlated to their ability to inhibit pathogens (8, 12) and protect humans from gastrointestinal diseases (28). Nowadays, foods fortified with health-promoting probiotic bacteria are mainly produced with fresh milk or milk derivatives such as yogurt, cheese, ice cream, and desserts (27). Cheeses with long ripening times, such as Cheddar, have also been manufactured using probiotic strains which multiply and survive throughout the ripening cycle without altering cheese quality (16, 42).
Functional food industries are now focusing on new nondairy foods that can contribute to a regular assumption of probiotics in individuals with lactose intolerance or with a diet lacking milk-derived products. The suitability of cereals as a substrate for growing probiotic bacterial strains has been reviewed by Charalampopoulos et al. (5) but at present only a few functional cereal-based products are available on the market, including oat meal gruel mixed with a fruit drink containing Lactobacillus plantarum 299v (30) and a rose hip drink with oats fermented by L. plantarum 299v (24, 30).
To colonize the gastrointestinal tract, probiotic strains need to be ingested as large populations and on a daily basis (35). Therefore, food manufacturers are trying to include probiotic strains in foods and beverages which are part of a normal diet to provide health defense while enjoying meals and to differentiate such functional products from concentrated probiotic preparations available as capsules, powders, or liquids. Research is currently under way to obtain a variety of probiotic products such as dry sausage fermented by Lactobacillus rhamnosus strains (14), fruit pieces containing Bifidobacterium spp. (G. Maguiña et al., Abstr. Annu. Meet. Food Expo., abstr. 15E-25, 2002), dried fruits vacuum-impregnated with Saccharomyces cerevisiae and Lactobacillus casei subsp. rhamnosus (3), soy milk fermented with Bifidobacterium breve (41), and an oat-based cereal bar including Bifidobacterium lactis (32).
The olive phylloplane, in particular the fruit surface, is suitable for the survival of microbial populations, in particular lactic acid bacteria (25, 31) which are involved in developing the spontaneous or started lactic fermentation of table olives (17). In the fermentation process of the most well-known preparation methods, i.e., Spanish-style green olives and California-style and Greek-style black olives as well as in the fermentation process of Sicilian green olives, the main microorganisms involved belong to the species Lactobacillus plantarum, Lactobacillus casei, and Leuconostoc mesenteroides (34, 43). Several studies have focused on the use of selected lactic acid bacteria to pilot a standardized fermentation process for olive production (2, 10), to control microbial spoilage (36, 39), to improve the fermentation process at low temperatures (13), and to reduce lye treatment for debittering green olives (6).
Our study aims to broaden the range of functional food types by exploitation of the microarchitecture of the olive surface and the nutritional qualities of olive pulp to develop a tasty, vegetable-based functional food consisting of table olives fortified with probiotic strains. The incorporation of health-promoting bacteria into table olives would add functional features to their current nutritional properties, e.g., antioxidant polyphenols having strong free-radical scavenging action and preventing atherogenesis (4, 46); vitamins (belonging to groups A, B, and E) and their precursors involved in the delay of cellular aging (17, 19); minerals such as potassium, magnesium, manganese, iron, calcium, vanadium and particularly sulfur (14 to 38 mg/100 g), as essential elements for the metabolism of sulfurous proteins (19); and an oil fraction (14 to 30%) basically consisting of monounsaturated fatty acids which are known to increase the levels of protective high-density lipoprotein cholesterol (46).
The ability of seven strains belonging to the probiotic species Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium bifidum, and Bifidobacterium longum to survive on the olive surface was investigated in five different table olive samples.
This study has indicated the suitability of table olives as a biological carrier for a selected strain which survived passage through the gastrointestinal tract and maintained colonization.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Lactobacillus rhamnosus ATCC 53103 (commercially named Lactobacillus GG), Bifidobacterium bifidum ATCC 15696, and Bifidobacterium longum ATCC 15708 were obtained from the American Type Culture Collection. Human isolates belonging to probiotic species, Lactobacillus rhamnosus IMPC11 and IMPC19 and Lactobacillus paracasei IMPC2.1 (deposited as strain LMG P-22043 in the Belgian Coordinated Collections of Microorganisms, Ghent, Belgium) and IMPC4.1 were obtained from the Culture Collection at the Microbiology Institute of the Catholic University of Piacenza, Italy.
Working cultures were prepared by inoculating de Man Rogosa Sharpe (MRS) broth (Difco, Detroit, Mich.) with L. rhamnosus and L. paracasei strains (37°C for 24 h) or MRS medium supplemented with 0.05% (wt/vol) l-cysteine-HCl (Sigma-Aldrich Division, Milan, Italy) (MRSC) with bifidobacteria (37°C for 48 h). L. rhamnosus and Bifidobacterium strains were grown anaerobically (Gas Pack System, Oxoid, Basingstoke, Hampshire, England), while L. paracasei strains were cultured in aerobic conditions. Working cultures were subcultured twice before use in experiments. The cell concentration of individual strains was evaluated by checking the optical density value at 600 nm (OD600) and then by plating diluted suspensions on agar plates.
For long-term storage, stock cultures were prepared by mixing 8 ml of a fresh culture with 2 ml of Bacto glycerol (Difco, Detroit, Mich.) and then freezing 1-ml aliquots of this mixture at −80°C in 2-ml sterile cryovials (Nalgene, Rochester, N.Y.).
Origin of the olive samples.
Commercial ready-to-eat table olives preserved in brine (about 4% wt/vol NaCl) were purchased from local supermarkets in Bari, Italy, while fresh olives were obtained from a local farm (Azienda Santo Stefano, Cerignola, Italy). In particular, one sample of fresh green olives (sample E, Bella di Cerignola variety, size 71 to 80 fruits/kg) and four commercial samples of different types of olives (samples A to D) were used. The information printed on the labels of samples A, B, C, and D is as follows: sample A: black pitted olives, Hojiblanca variety, Alisa brand, size 371 to 400 fruits/kg, preserved in brine (pH 6.3) containing ferrous gluconate (residue), packed in 425 ml cans, and heat sterilized; sample B: black olives, Bella di Cerignola variety, Belaria brand, size 71 to 80 fruits/kg, preserved in brine (pH 5.1) containing ferrous gluconate (residue) and lactic acid, and packed in 4,700-ml cans; sample C: Spanish black pitted olives, Hojiblanca variety, Ponti brand, size 371 to 400 fruits/kg, preserved in brine (pH 6.5) containing ferrous gluconate (residue), packed in 425-ml cans, and heat sterilized; and sample D: green pitted olives, Hojiblanca variety, Saclà brand, size 371 to 400 fruits/kg, preserved in brine (pH 3.9) containing lactic acid and ascorbic acid, and packed in 314-ml glass jars.
Commercial brands of samples A, B, C, and D were selected from among the most common table olives marketed in Italy. Olives were used without any further handling prior to the experiments. All commercial samples (olives and brines) were checked for the absence of microbial population (sterility) as described below under Microbiological analysis.
Inoculum of bacterial strains.
Olive samples A, B, C, and D (about 240 g) were placed in 500-ml sterile glass jars and covered with their own brine, while fresh olives from sample E (about 240 g) were washed in tap water to eliminate superficial dirt deposits, dipped in sterile brine (4% wt/vol NaCl), and placed in jars. Olive samples (2 jars each) were separately inoculated with 10 ml of a bacterial suspension containing about 5 × 1013 CFU for lactobacilli or about 5 × 1011 CFU for bifidobacteria. Cell concentrations of approximately 1011 to 109 CFU ml−1 of olive brine were obtained. Bacterial suspensions were prepared by inoculating 2 ml of working culture of individual strains into 100 ml MRS or MRSC for lactobacilli and bifidobacteria, respectively. After growth in the conditions described above, cells were harvested by centrifugation (5,000 × g, 10 min, 4°C), washed with 50 mM phosphate buffer (pH 6.5), and suspended in 10 ml of sterile distilled water. Inoculated samples were stored at room temperature (about 25°C) for 3 months. The experiments were performed twice (total number of measurements, four).
Lactobacillus GG, a strain well known for its probiotic characteristics (40), was used to select the most suitable olive sample for hosting probiotic species. Ten ml of the bacterial suspension prepared as described above was added to each jar containing olives of samples A, B, C, D, or E.
On the basis of survival performance observed for Lactobacillus GG on different samples, olive sample C was selected as the most suitable to evaluate the survival of other probiotic species, L. rhamnosus IMPC11 and IMPC19, L. paracasei IMPC2.1 and IMPC4.1, B. bifidum ATCC 15696, and B. longum ATCC 15708. Ten ml of each bacterial suspension (about 5 × 1013 or 5 × 1011 CFU for lactobacilli and bifidobacteria, respectively) was separately added to jars. In the case of bifidobacteria, jars were supplemented with 0.025% (wt/vol) l-cysteine-HCl.
Microbiological analysis.
For microbiological analysis, 10 ml of brine and four olives were taken from each jar at inoculation time and after 7, 14, 21, 30, and 90 days of incubation at room temperature. Olives and brines of samples A, B, C, D, and E without bacterial inoculum (control jars), stored in the same conditions as the inoculated samples, were also subjected to microbiological analysis at the same sampling times. Olives were drained, transferred aseptically to sterile tubes containing 20 ml of sterile NaCl (0.85%, wt/vol) solution supplemented with Tween 80 (0.025%, vol/vol), and shaken vigorously for 2 h. The resulting suspensions were serially diluted and plated in duplicate on MRS or MRSC agar for counting purposes. In addition, at inoculation time suspensions were plated on different media, Slanetz & Bartley agar (SB) (Oxoid), Rogosa SL agar (Difco), potato dextrose agar (Difco), plate count agar (Difco), MRS agar, and MRSC agar, to verify the sterility of commercial samples (A, B, C, and D) or to detect indigenous microflora on fresh olives (sample E).
Discrimination of Lactobacillus GG from indigenous microflora on fresh olives.
Lactobacillus GG was discriminated from indigenous microflora occurring in sample E by the morphological characteristics of colonies detected on MRS agar plates and by differentiating the cell wall protein profile of isolates. Colonies with morphological characteristics corresponding to that of Lactobacillus GG were defined as GG-like colonies. Twenty percent of total colonies detected on countable agar plates from sample E and from the corresponding noninoculated control were randomly picked at each sampling time and checked for purity; then the morphological characteristics of the colonies and the corresponding cell wall protein profiles were compared. Therefore, the number of Lactobacillus GG cells surviving during the experiment was obtained by counting the GG-like colonies, while the remaining colonies were indicative of indigenous microflora.
For cell wall protein analysis, isolates were grown overnight in MRS broth at 37°C, then the cultures were centrifuged (5,000 × g, 10 min, 4°C), and the cells were washed twice in 50 mM Tris-HCl, pH 7.5, containing 0.1 M CaCl2. After centrifugation at 8,000 × g for 5 min, cell wall proteins were extracted using the method previously reported (18) with minor modifications (9) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in an Agilent 2100 Bioanalyzer (Agilent Technologies GmbH, Waldbronn, Germany). The chip-based protein analysis was performed according to the manufacturer's protocol using a Protein 200 Plus LabChip kit and dedicated Protein 200 Plus assay software. The sizing ladder consisted of 11 different proteins, including the system peak and two internal markers ranging from 14.4 to 200 kDa.
Recovery of L. paracasei IMPC2.1 from human feces after olive intake.
Ten to 15 table olives from sample C, carrying about 109 to 1010 CFU of L. paracasei IMPC2.1, were administered to five healthy volunteers, two male and three female, aged 38 to 64 years. The strain, deposited as strain LMG P-22043 in the Belgian Coordinated Collections of Microorganisms, was previously selected for its potential probiotic characteristics assessed in vitro, e.g., high tolerance to gastric juice, NaCl, and bile salt and adhesion to pig mucus (P. Lavermicocca et al., patent application EP2004/013582). Informed consent was obtained from all volunteers, and they were asked to abstain from consumption of products containing lactobacilli 1 week before and during the whole experiment. Fecal samples were collected at the start and the end of intervention (day 10) and at the end of the follow-up (day 17).
At each sampling, microbial analysis and reisolation of the strain were done as follows. Fecal samples were suspended (1:10 wt/vol) in liquid AMIES (BD Italia, Milan, Italy) and 10-fold serially diluted and 100 μl of appropriate dilutions was plated on SL-Rogosa agar (Difco Laboratories, Detroit, Mich.) with or without 12 μg ml−1 of vancomycin (Sigma-Aldrich Division, Milan, Italy). Vancomycin-resistant lactobacilli, including L. paracasei, were enumerated on SL-Rogosa-vancomycin agar. Plates were incubated for 3 days at 37°C in anaerobic jars.
Genetic identification of L. paracasei IMPC2.1 strain.
PCR- amplified ribosomal DNA restriction analysis (ARDRA) was performed according to Ventura et al. (44) for the identification of L. paracasei among the vancomycin-resistant Lactobacillus species. Briefly, 10 to 20% of total colonies randomly selected from countable SL-Rogosa-vancomycin agar plates were isolated and checked for purity and DNA was extracted using a microlysis kit (Microzone Ltd., United Kingdom). The 16S rRNA gene was obtained by amplification and analyzed using the ARDRA technique. The Sau3AI restriction enzyme was used for discriminating L. paracasei from the other vancomycin-resistant species. In order to sort out the IMPC2.1 strain from the L. paracasei identified by ARDRA, a repetitive extragenic palindromic-PCR (REP-PCR) analysis was performed according to the method of Hyytiä-Trees et al. (21). Two degenerate primers, REP-1R-Dt (5′-IIINCGNCGNCATTCNGGC-3′) (where N is A, T, C, or G and I is inosine) and REP-2R-Dt (5′-NCGNCTTATCNGGCCTAC-3′), were used (45). The amplifications were performed in a GeneAmp PCR system 9700 (Perkin-Elmer) following the PCR conditions described by Hyytiä-Trees et al. (21).
Scanning electron microscopy.
Scanning electron microscopy observations were performed to assess the ability of strains used in this study to adhere to the olive surface. Samples were prepared by using a scalpel to cut the olive skin, which was fixed in 2.5% (vol/vol) glutaraldehyde in 100 mM cacodylate buffer (pH 7.4) and rinsed three times in cacodylate buffer (pH 7.4). All chemicals were obtained from Fluka (Sigma-Aldrich Division, Milan, Italy). Samples were dehydrated with a graded series of ethanol (75, 85, 95, and 100%) (33), dried at the critical point of liquid CO2, and then coated with gold. Olive skins were observed with a Philips XL 30 ESEM scanning microscope (Philips, Hillsboro, Oregon) at an acceleration voltage of 25 kV.
Statistical analyses.
For the microbiological analyses, mean CFU ± standard error was calculated for each experiment. Data were analyzed by one-way analysis of variance followed by the Fisher test using STATISTICA 6.0 software (StatSoft software package, Tulsa, OK). A P value of <0.05 was accepted as statistically significant.
RESULTS
Survival of bacterial strains on olives.
Neither commercial products (samples A, B, C, and D) nor noninoculated control jars stored over the 3-month experimental period yielded bacterial colonies on agar media from olives and brines. The appearance, texture, and organoleptic quality of products resulting from the addition of probiotic species were identical to those of the commercial products.
Lactobacillus GG survival.
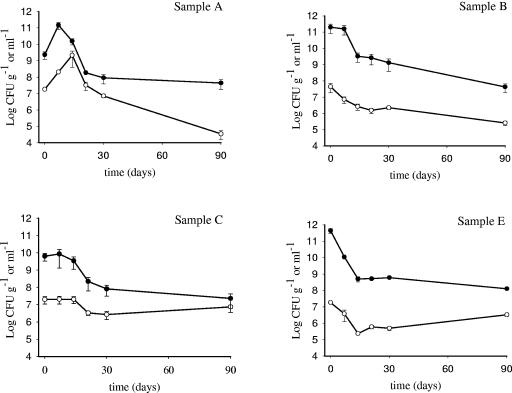
Bacterial survival was observed on four out of five olive samples. On olive sample D, bacterial cells survived for 1 week, possibly due to the low pH (3.9) of the olive brine; the number of viable cells after a week dropped to 0 from 3.7 × 106 CFU g−1 on olives and 109 CFU ml−1 in brines. The behavior of Lactobacillus GG survival on the remaining four olive samples and in the corresponding brines is shown in Fig. 1. On olive samples A and B the number of viable cells declined during incubation. On the former, the number of viable cells was 1.8 × 107 CFU g−1 at inoculation time and significantly (P < 0.05) increased during the first 14 days (2.1 × 109 CFU g−1), then dropped to 3.4 × 104 CFU g−1 after 90 days (P < 0.05). On sample B, Lactobacillus GG significantly (P < 0.05) dropped from 4.4 × 107 to 2.5 × 105 CFU g−1 after 3 months. Conversely, no great fluctuation in the number of bacterial CFU during the entire experiment was observed with Lactobacillus GG on olive sample C: 2 × 107 CFU g−1 were recovered after inoculation and the number was not significantly different after 90 days (7.4 × 106 CFU g−1) (P > 0.05) (Fig. 1). Therefore, olive sample C was selected to investigate the survival of the other strains.
FIG. 1.
Behavior of L rhamnosus GG survival on olives (○) and in brines (•) from olive samples A, B, C, and E. Data, expressed as means ± standard errors, are from two independent experiments with two replicates each (n = 4). Mean counts of L. rhamnosus GG from sample E were obtained by enumerating the GG-like colonies.
To evaluate the ability of Lactobacillus GG to colonize fresh olives, its survival behavior was assessed on olive sample E (Fig. 1). Microbiological analysis of the inoculated sample and the corresponding control indicated the presence of bacterial colonies with different morphologies, such as GG-like colonies, detected only in the inoculated sample, and indigenous microflora. Lactobacillus GG forms large, creamy white colonies on MRS agar that are generally distinct from other lactic acid bacterial colonies (1).
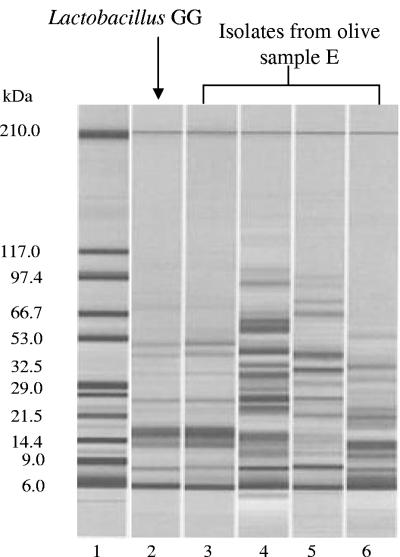
In order to confirm the presence of Lactobacillus GG detected by colony morphology, a cell wall protein profile analysis of isolates was performed on 20% of total colonies randomly picked from countable agar plates. The analysis, performed at each sampling time, showed three representative profiles in both control and inoculated samples, while one additional profile, corresponding to the GG-like colonies, was observed only in the inoculated sample. As an example, at 14 days, the electrophoretic pattern obtained from the analysis of 10 colonies isolated from a plate of the inoculated sample containing a total of about 50 (dilution factor 104) colonies, is reported in Fig. 2. Three colonies screened as GG-like out of 10 evaluated showed the protein profile reported in lane 3, which was comparable to that of the reference strain Lactobacillus GG (lane 2); protein fingerprints reported in lanes 4, 5, and 6 can be ascribed to indigenous bacterial colonies observed both in the control and in the inoculated sample.
FIG. 2.
Gel-like image of cell wall protein profiles of representative colonies recovered from olive sample E at 14 days of sampling. Lane 1, Protein 200 Plus ladder; lane 2, reference strain Lactobacillus GG; lane 3, GG-like colony recovered from olives; lanes 4 to 6, other colonies recovered from olives.
Additionally, the results of cell wall protein profile analysis performed comparing another seven GG-like colonies picked from the same agar plate to the reference strain L. rhamnosus GG were in agreement with the screening based on colony morphology (data not shown). Results showed that viable Lactobacillus GG cells declined considerably on olives during the first 14 days from 1.8 × 107 to 2.3 × 105 CFU g−1 (P < 0.05), and a survival of 3.2 × 106 CFU g−1 was recorded after 90 days. Few indigenous microbial colonies were observed on SB, plate count agar, and potato dextrose agar at inoculation time, while after 14, 21, 30, and 90 days about 105 to 106 CFU per gram of olives were recovered on MRS agar.
The behavior of the bacterial strain in brines (Fig. 1) reflected the survival performance observed on olives, with the number of viable cells recovered in brines generally being 100- to 1,000-fold higher than on the fruits themselves.
Survival of lactobacilli and bifidobacteria.
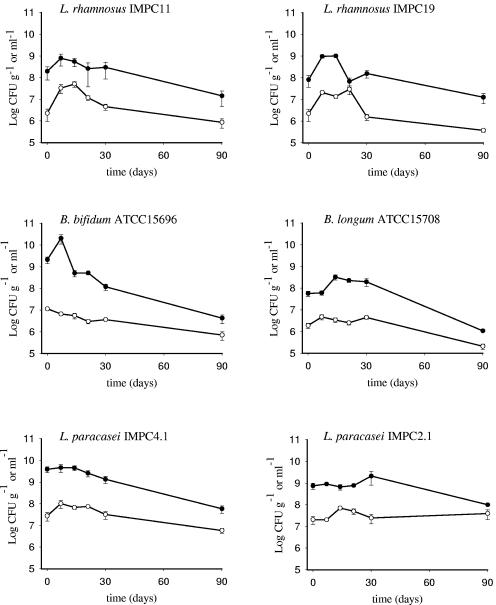
L. rhamnosus IMPC11 and IMPC19, B. longum ATCC 15708, B. bifidum ATCC 15696, and L. paracasei IMPC2.1 and IMPC4.1 survived on olive sample C over a 3-month storage period, as shown in Fig. 3. A significant increase was observed in populations of L. rhamnosus IMPC11 and IMPC19 (from 2.3 × 106 to 3.3 × 107 CFU g−1 and from 2.2 × 106 to 2.1 × 107 CFU g−1, respectively) during the first 7 days (P < 0.05), while the number of viable cells declined to 4.5 × 106 and 1.5 × 106 CFU g−1, respectively, after 30 days and remained almost unvaried until the end of the sampling period (P > 0.05) (Fig. 3). A good survival rate until 30 days (more than 106 CFU g−1) was observed in the case of bifidobacteria populations: about 1.1 × 107 CFU g−1 was recovered for B. bifidum at inoculation time; afterwards the cell concentration declined (P < 0.05) till 30 days (3.6 × 106 CFU g−1), with a final recovery of 7 × 105 CFU g−1 after 90 days (P < 0.05). Similar behavior was observed for B. longum, which declined to 2.1 × 105 CFU g−1 after 90 days.
FIG. 3.
Survival of L. rhamnosus IMPC11 and IMPC19, B. bifidum ATCC 15696, B. longum ATCC 15708, and L. paracasei IMPC4.1 and IMPC2.1 on olive sample C (○) and in the corresponding brine (•). Data, expressed as means ± standard errors, are from two independent experiments with two replicates each (n = 4).
With regard to the L. paracasei species, strains IMPC4.1 and IMPC2.1 maintained high viability on olives (Fig. 3). Particular attention should be addressed to the high survival throughout the experiment of L. paracasei IMPC2.1, with more than 107 viable cells per gram of olives recovered at each sampling time (P > 0.05). For all strains, cells survived in brines to a greater extent (generally 10- to 100-fold) than on olives (Fig. 3).
Recovery of L. paracasei IMPC2.1 from human feces after olive intake.
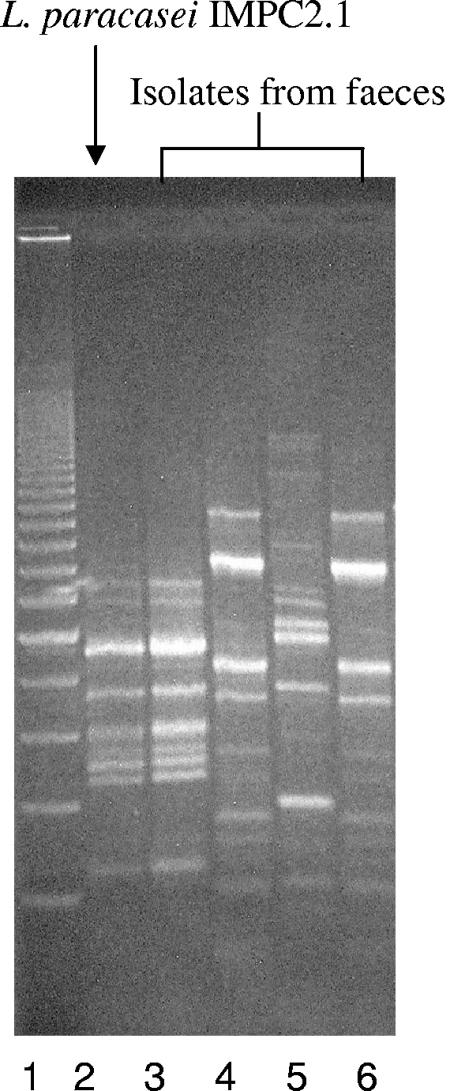
This test was carried out to validate table olives as a carrier for transporting bacterial cells into the human gastrointestinal tract. This was demonstrated by the recovery of L. paracasei IMPC2.1 (LMG P-22043) from four out of five fecal samples (collected at the end of the consumption period) of volunteers fed 10 to 15 olives carrying about 109 to 1010 CFU (Table 1), while the strain was not detected in any of the feces samples obtained before the consumption of olives. The REP-PCR profiles obtained from colonies isolated from the fecal sample of volunteer 3 at day 10 are shown in Fig. 4: the profile reported in lane 3 was identical to the pattern obtained from L. paracasei IMPC2.1, used as a positive control (lane 2). The strain was also still identified in one of the five volunteers at the end of follow-up (day 17) (Table 1).
TABLE 1.
Total vancomycin-resistant Lactobacillus count in the feces of five healthy volunteers fed L. paracasei IMPC2.1 (LMG P-22043)-containing olives and recovery of the strain identified by PCR
| Day of samplinga | Subject no. | Lactobacillus spp.b (CFU g−1, wet wt) | No. of colonies on platec | No. of L. paracasei IMPC2.1 colonies/no. analyzed |
|---|---|---|---|---|
| 0 | 1 | 7.0 × 104 | 6 × 103 | 0/2 |
| 2 | 2.7 × 107 | 24 × 105 | 0/3 | |
| 3 | 1.6 × 106 | 11 × 104 | 0/2 | |
| 4 | 1.8 × 107 | 16 × 105 | 0/3 | |
| 5 | 1.1 × 106 | 10 × 104 | 0/3 | |
| 10 | 1 | 3.1 × 106 | 24 × 104 | 3/5 |
| 2 | 5.7 × 108 | 26 × 107 | 1/3 | |
| 3 | 5.1 × 105 | 47 × 103 | 1/4 | |
| 4 | 6.7 × 106 | 21 × 104 | 1/3 | |
| 5 | 8.3 × 105 | 15 × 104 | 0/3 | |
| 17 | 1 | 5.2 × 105 | 48 × 103 | 0/5 |
| 2 | 4.5 × 106 | 7 × 105 | 1/2 | |
| 3 | 3.3 × 105 | 26 × 103 | n.a.d | |
| 4 | 5.7 × 105 | 43 × 103 | n.a. | |
| 5 | 2.0 × 106 | 13 × 104 | 0/3 |
Day 0, before consumption; day 10, end of consumption; day 17, 1 week after end of consumption. The daily dose (total CFU) was 3.1 × 1010 CFU (subjects 1 and 2) or 4.0 × 109 CFU (subjects 3, 4, and 5).
Vancomycin resistant.
Number of colonies × dilution factor.
n.a., not analyzed.
FIG. 4.
Detection of Lactobacillus paracasei IMPC2.1 by REP-PCR. Lane 1, 200-bp DNA step ladder (Promega); lane 2, reference strain L. paracasei IMPC2.1; lanes 3 to 6, strains from fecal sample of subject number 3 at day 10: lane 3, strain IMPC2.1; and lanes 4 to 6, other strains.
Adhesion of bacterial cells to the olive surface.
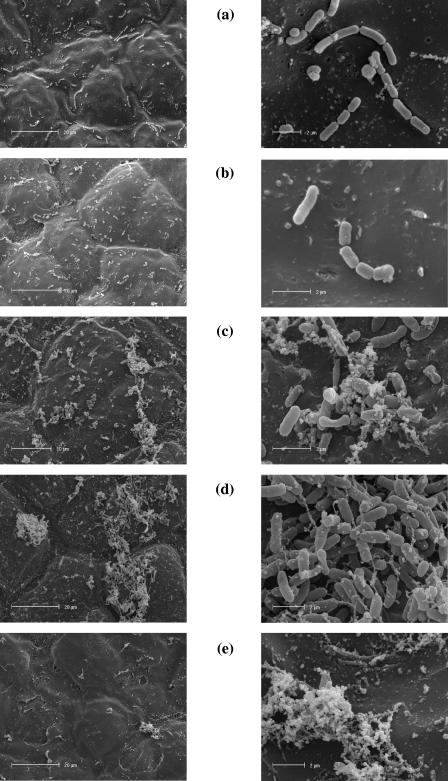
Electron microscopic observation of the surface of olive sample C revealed a regular distribution of bacterial cells for L. rhamnosus and L. paracasei strains, while bifidobacterial cells tended to aggregate (Fig. 5). In particular, B. longum ATCC 15708 (Fig. 5c) seemed to adhere to the olive surface through small aggregates of amorphous material occurring on the control (Fig. 5e), while B. bifidum ATCC 15696 cells appeared to cluster directly on the olive surface (Fig. 5d).
FIG. 5.
Colonization of bacterial cells on the surface of olive sample C: (a) Lactobacillus GG; (b) L. paracasei IMPC2.1; (c) B. longum ATCC 15708; (d) B. bifidum ATCC 15696; (e), noninoculated olive surface.
DISCUSSION
The results reported here suggest that table olives are a suitable substrate for delivering probiotic species, since populations of L. paracasei IMPC2.1 (LMG P-22043), a strain selected for its potential probiotic characteristics assessed in vitro and for its lengthy survival on olives, were detected in the feces of human volunteers after daily consumption of olives containing about 109 to 1010 viable cells. This result meets one of the aims of the current research, that of finding new delivery systems ensuring the stability and viability of strains (29). The experimental results obtained in our study using olive sample C show that both strains of L. paracasei survived the 3-month experiments better than the other species tested. In particular, a moderate fluctuation around 107 CFU g−1 was recorded for this species throughout the experiment.
The bifidobacteria and L. rhamnosus strains showed a good survival rate until 30 days (more than 106 CFU g−1); afterwards the population remained unvaried for L. rhamnosus strains, while a slight decline was observed for bifidobacteria (Fig. 1 and 3). The survival performance observed for bifidobacteria is quite promising for a successful introduction of these species into the vegetable food chain. It is noteworthy that incorporation of this group of bacteria into dairy products has proved to be difficult due to their sensitivity to the environmental conditions of fermented products (low pH, long storage periods at low temperatures, oxygen, interactions with lactic acid bacteria starters) (20). Experiments to improve the technological performance and gastrointestinal survival of bifidobacteria have been performed by incorporating microbial cells into calcium alginate beads or granular starch (7, 26). Generally, microbial cells are immobilized in food matrices through attachment to a solid substrate (15). In the case of table olives, scanning electron microscopic observation revealed that the fruit surface is an example of microarchitecture where bacteria can survive by adhering to skin (Fig. 5). Similar observations were reported by Nychas et al. (31), who indicated epicuticular waxes as the site of attachment for colonization by yeasts and bacteria.
In conclusion, table olives might be a good candidate for producing a novel and tasty functional vegetable food, also due to the storage temperature of the product; the level of viable probiotic strains ranged from 107 to 108 CFU g−1 during storage at room temperature for Lactobacillus GG and L. paracasei strains. This means that, considering an intake of about 10 to 15 olives per portion (corresponding to 30 to 50 g of pulp), an amount ranging from 108 to 109 CFU of viable probiotic strains could be ingested using preparations stored for 3 months at room temperature. These amounts are comparable to those of milk-based probiotic products, e.g., bioyogurt, containing more than 106 probiotic bacteria per ml at the end of their shelf life, which does not exceed 30 days when stored under refrigeration (27).
Further studies should be addressed to assessing the contribution of probiotic strains to lactic fermentation performed by starter strains. In this regard, the survival of Lactobacillus GG on fresh olives from sample E in the presence of indigenous bacteria (Fig. 1) is of particular interest. The entity of the final colonization of the probiotic strain indicates that its survival could be improved by the presence of other bacterial species. This has technological implications when coinoculation of starters and probiotic strains is carried out. Among the strains evaluated in this study, L. paracasei IMPC2.1 (LMG P-22043) could be considered an example of a probiotic strain suitable for olive processing, also in light of its strict taxonomical relationship with bacterial species, e.g., Lactobacillus casei, involved in the natural fermentation of table olives (34).
Finally, this research suggests that a vegetable food matrix, such as olives, might be an efficient vehicle for administering probiotics in a tasty, nonrefrigerated functional product.
Acknowledgments
We thank Anthony Green for help in the language revision.
REFERENCES
- 1.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Samela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balatsouras, G., V. Papamichael, and N. Eldin. 1971. Fermentation of green olives by thermophilic lactic acid bacteria at a temp. of 37-40 degree. Grasas Aceites 22:357-366. [Google Scholar]
- 3.Betoret, N., L. Puente, M. J. Díaz, M. J. García, M. L. Gras, J. Martínez-Monzó, and P. Fito. 2003. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 56:273-277. [Google Scholar]
- 4.Carluccio, M. A., L. Siculella, M. A. Ancora, M. Massaro, E. Scoditti, C. Storelli, F. Visioli, A. Distante, R. De Caterina. 2003. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation. Arterioscler. Thromb. Vasc. Biol. 23:622-629. [DOI] [PubMed] [Google Scholar]
- 5.Charalampopoulos, D., R. Wang, S. S. Pandiella, and C. Webb. 2002. Application of cereals and cereal components in functional foods: a review. Int. J. Food Microbiol. 79:131-141. [DOI] [PubMed] [Google Scholar]
- 6.Ciafardini, G., V. Marsilio, B. Lanza, and N. Pozzi. 1994. Hydrolysis of oleuropein by Lactobacillus plantarum strains associated with olive fermentation. Appl. Environ. Microbiol. 60:4142-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crittenden, R., A. Laitila, P. Forssell, J. Mättö, and M. Saarela. 2001. Adhesion of bifidobacteria to granular starch and its implication in probiotic technologies. Appl. Environ. Microbiol. 67:3469-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross, M. L. 2002. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol. Med. Microbiol. 34:245-253. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis, M., A. Corsetti, N. Tosti, J. Rossi, M. R. Corbo e M. Gobbetti. 2001. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 67:2011-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deiana, P., G. A. Farris, P. Catzeddu, and G. Madau. 1992. Impiego di fermenti lattici e lieviti nella preparazione delle olive da mensa. Ind. Aliment. XXXI:1011. [Google Scholar]
- 11.de Roos, M., and M. B. Katan. 2000. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 71:405-411. [DOI] [PubMed] [Google Scholar]
- 12.Drago, L., M. R. Gismondo, A. Lombardi, C. de Haën, and L. Gozzini. 1997. Inhibition of in vitro growth of enteropathogens by new Lactobacillus isolates of human intestinal origin. FEMS Microbiol. Lett. 153:455-463. [DOI] [PubMed] [Google Scholar]
- 13.Duran Quintana, M. C., P. Garcýa Garcýa, and A. Garrido Fernandez. 1999. Establishment of conditions for green table olive fermentation at low temperature. Int. J. Food Microbiol. 51:133-143. [DOI] [PubMed] [Google Scholar]
- 14.Erkkilä S. M. L. Suihko, S. Eerola, E. Petäjä, and T. Mattila-Sandholm. 2001. Dry sausage fermented by Lactobacillus rhamnosus strains. Int. J. Food Microbiol. 64:205-210. [DOI] [PubMed] [Google Scholar]
- 15.Fleet, G. H. 1999. Microorganisms in food ecosystems. Int. J. Food Microbiol. 50:101-117. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner, G., R. P. Ross, J. K. Collins, G. Fitzgerald, and C. Stanton. 1998. Development of a probiotic cheddar cheese containing human-derived Lactobacillus paracasei strains. Appl. Environ. Microbiol. 64:2192-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido Fernandez, A., M. J. Fernandez Diez, and M. R. Adams. 1997. Physical and chemical characteristics of the olive fruit, p. 67-109. In A. Garrido Fernandez et al. (ed.), Table olives. Chapman & Hall, London, United Kingdom.
- 18.Gatti, M., E. Fornasari, and E. Neviani. 1997. Cell-wall protein profiles of dairy thermophilic lactobacilli. Lett. Appl. Microbiol. 25:345-348. [DOI] [PubMed] [Google Scholar]
- 19.Giomo, A. 2001. Dossier oliva da mensa. Una risorsa da scoprire. Olivo Olio 9:20-23. [Google Scholar]
- 20.Gobbetti, M., A. Corsetti, E. Smacchi, A. Zocchetti, and M. De Angelis. 1998. Production of crescenza cheese by incorporation of bifidobacteria. J. Dairy Sci. 81:37-47. [Google Scholar]
- 21.Hyytiä-Trees, E., U. Lyhs, H. Korkeala, and J. Björkroth. 1999. Characterisation of ropy slime-producing Lactobacillus sakei using repetitive element sequence-based PCR. Int. J. Food Microbiol. 50:215-219. [Google Scholar]
- 22.Isolauri, E., Y. Sütas, P. Kankaanpää, H. Arvilommi, and S. Salminen. 2001. Probiotics: effects on immunity. Am. J. Clin. Nutr. 73(Suppl.):444s-450s. [DOI] [PubMed] [Google Scholar]
- 23.Jahreis, G., H. Vogelsang, G. Kiessling, R. Schubert, C. Bunte, and W. P. Hammes. 2002. Influence of probiotic sausage (Lactobacillus paracasei) on blood lipids and immunological parameters of healthy volunteers. Food Res. Int. 35:133-138. [Google Scholar]
- 24.Johansson M.-L., S. Nobaek, A. Berggren, M. Nyman, I. Björck, S. Ahrné, and B. Jeppsson. 1998. Survival of Lactobacillus plantarum DSM 9843 (299v), and effect on the short-chain fatty acid content of faeces after ingestion of a rose-hip drink with fermented oats. Int. J. Food Microbiol. 42:29-38. [DOI] [PubMed] [Google Scholar]
- 25.Lavermicocca, P., M. Gobbetti, A. Corsetti, and L. Caputo. 1998. Characterization of lactic acid bacteria isolated from olive phylloplane and table olive brines. Ital. J. Food Sci. 10:27-39. [Google Scholar]
- 26.Lee, K. Y., and T. R. Heo. 2000. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juice and bile salt solution. Appl. Environ. Microbiol. 66:869-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lourens-Hattingh, A., and B. C. Viljoen. 2001. Yogurt as probiotic carrier food. Int. Dairy J. 11:1-17. [Google Scholar]
- 28.Marteau, P. R., M. de Vrese, C. J. Cellier, and J. Schrezenmeir. 2001. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 73(Suppl.):430s-436s. [DOI] [PubMed] [Google Scholar]
- 29.Mattila-Sandholm, T., P. Myllärinen, R. Crittenden, G. Mogensen, R. Fondén e M. Saarela. 2002. Technological challenges for future probiotic foods. Int. Dairy J. 12:173-182. [Google Scholar]
- 30.Molin, G. 2001. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73(Suppl.):380s-385s. [DOI] [PubMed] [Google Scholar]
- 31.Nychas G.-J.E., E. Z. Panagou, M. L. Parker, K. W. Waldron, and C. C. Tassou. 2002. Microbial colonization of naturally black olives during fermentation and associated biochemical activities in the cover brine. Lett. Appl. Microbiol. 34:173-177. [DOI] [PubMed] [Google Scholar]
- 32.Ouwehand, A. C., T. Kurvinen, and P. Rissanen. 2004. Use of probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int. J. Food Microbiol. 95:103-106. [DOI] [PubMed] [Google Scholar]
- 33.Piva, A., A. Prandini, L. Fiorentini, M. Morlacchini, F. Galvano, and J. B. Luchansky. 2002. Tributyrin and lactitol synergistically enhanced the trophic status of the intestinal mucosa and reduced istamine levels in the gut of nursery pigs. J. Anim. Sci. 80:670-680. [DOI] [PubMed] [Google Scholar]
- 34.Randazzo, C. L., C. Restuccia, A. D. Romano, and C. Caggia. 2004. Lactobacillus casei, dominant species in naturally fermented Sicilian green olives. Int. J. Food Microbiol. 90:9-14. [DOI] [PubMed] [Google Scholar]
- 35.Reid, G., J. Howard, and B. S. Gan. 2001. Can bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Barba, J. L., D. P. Cathcart, P. J. Warner, and R. Jimenez-Diaz. 1994. Use of Lactobacillus plantarum LPCO10, a bacteriocin producer, as a starter culture in Spanish-style green olive fermentations. Appl. Environ. Microbiol. 60:2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saarela, M., L. Lähteenmäki, R. Crittenden, S. Salminen, and T. Mattila-Sandholm. 2002. Gut bacteria and health foods -the European perspective. Int. J. Food Microbiol. 78:99-117. [DOI] [PubMed] [Google Scholar]
- 38.Salminen, S., A. C. Ouwehand, and E. Isolauri. 1998. Clinical applications of probiotic bacteria. Int. Dairy J. 8:563-572. [Google Scholar]
- 39.Sanchez, A. H., L. Rejano, A. Montaño, and A. de Castro. 2001. Utilization at high pH of starter cultures of lactobacilli for Spanish-style green olive fermentation. Int. J. Food Microbiol. 67:115-122. [DOI] [PubMed] [Google Scholar]
- 40.Saxelin, M. 1997. Lactobacillus GG -a human probiotic strain with thorough clinical documentation. Food Rev. Int. 13:293-313. [Google Scholar]
- 41.Shimakawa, Y., S. Matsubara, N. Yuki, M. Ikeda, and F. Ishikawa. 2003. Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int. J. Food Microbiol. 81:131-136. [DOI] [PubMed] [Google Scholar]
- 42.Stanton C., G. Gardiner, P. B. Lynch, J. K. Collins, G. Fitzgerald, and R. P. Ross. 1998. Probiotic cheese. Int. Dairy J. 8:491-498. [Google Scholar]
- 43.Vaughn, R. H. 1954. Lactic acid fermentation of cucumbers, sauerkraut and olives, p. 417-478. In L. A. Underkofler and R. J. Hickey (ed.), Industrial fermentations. Chemical Publishing Co., New York, N.Y.
- 44.Ventura, M., I. A. Casas, L. Morelli, and M. L. Callegari. 2000. Rapid amplified ribosomal DNA restriction analysis (ARDRA) identification of Lactobacillus spp. isolated from faecal and vaginal samples. Syst. Appl. Microbiol. 23:504-509. [DOI] [PubMed] [Google Scholar]
- 45.Versalovic, J., M. Schneider, F. J. De Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 46.Visioli, F., A. Poli, and C. Galli. 2002. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 22:65-75. [DOI] [PubMed] [Google Scholar]