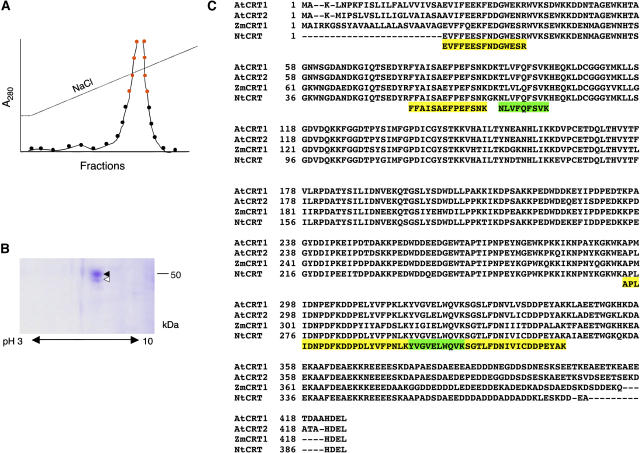
Figure 1.
Purification and identification of TMV MP-interacting protein. A, Purification by affinity chromatography. Protein amounts in the fractions eluted by the indicated continuous NaCl gradient were measured by A280. Fractions pooled for subsequent analysis by two-dimensional gel electrophoresis are indicated by red circles. B, Coomassie blue-stained two-dimensional gel of the pooled fractions indicated in A. Molecular mass expressed in thousands of Daltons is indicated on the right. C, Amino acid sequence of six peptides derived from the purified TMV MP-interacting protein and its alignment with full-length calreticulin sequences from Arabidopsis (AtCRT1, accession no. NM_104513.2, and AtCRT2, accession no. NM_100791.2), maize (ZmCRT1, accession no. Z46772.1), and tobacco (NtCRT, accession no. X85382.1). Alignment was performed by the Clustal algorithm; gaps introduced for alignment are indicated by dashes. Yellow and green boxes indicate peptide sequences derived from the top and bottom protein spots indicated by black and white arrowheads, respectively, in B.