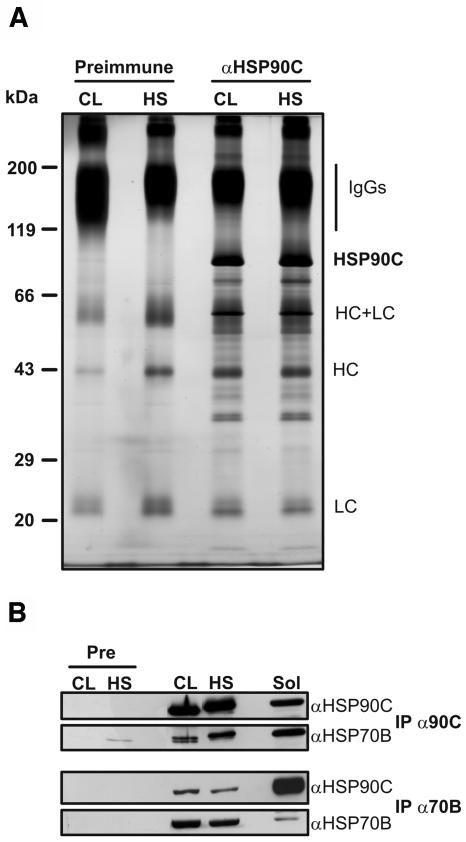
Figure 5.
Immunoprecipitation of HSP90C. A, Chlamydomonas total soluble proteins were prepared from cells grown in continuous light (CL) and from cells that, in the light, were exposed for 1 h to a heat shock (HS) at 40°C. Next, soluble proteins were incubated with protein A Sepharose coupled to antibodies of preimmune serum (Preimmune) or to affinity-purified anti-HSP90C antibodies. Immunoadsorbed proteins were eluted in nonreducing SDS sample buffer, separated on a 7.5% to 15% SDS-polyacrylamide gel, and visualized by silver staining. The positions of precipitated HSP90C and of contaminating IgG heavy chains (HC) and light chains (LC) are indicated. B, Seven hundred microliters of soluble lysate from approximately 109 cells generated as described in A were incubated with protein A Sepharose coupled to antibodies of preimmune (Pre), anti-HSP90C (IP α90C), or anti-HSP70B (IP α70B) serum. Fifteen microliters of the lysate (Sol) and one-third of each immunoprecipitation were separated on a 7.5% to 15% SDS-polyacrylamide gel, transferred to nitrocellulose, and immunodecorated with antibodies against HSP90C and HSP70B.