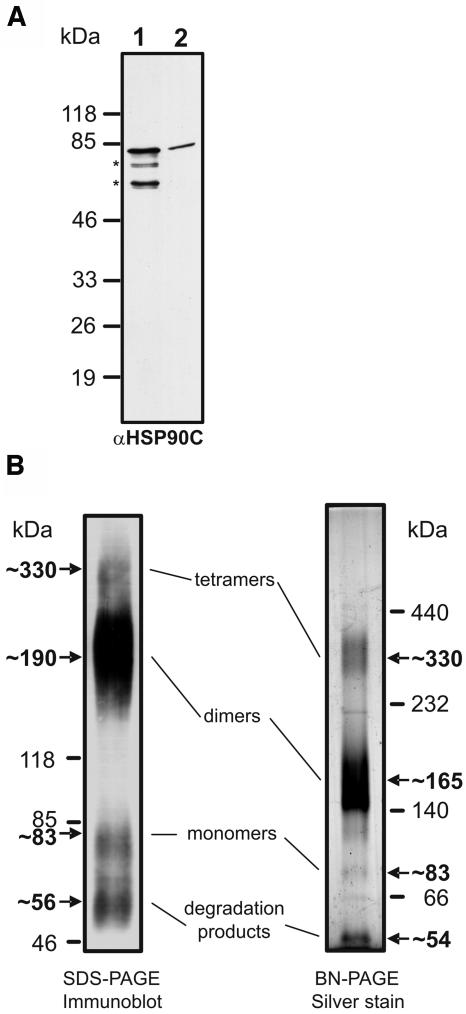
Figure 7.
Analysis of migration and oligomerization properties of heterologously expressed HSP90C. A, Fifty nanograms of heterologously expressed HSP90C lacking the putative 71-amino-acid transit peptide (lane 1) were separated on a 20-cm-long 7.5% to 15% SDS-polyacrylamide gel next to 50 μg of whole-cell Chlamydomonas proteins (lane 2). Proteins were transferred to nitrocellulose and immunodecorated with the HSP90C antibody. B, Four micrograms of heterologously expressed HSP90C were cross-linked with 0.1% glutaraldehyde for 10 min at 30°C, separated on a 4% to 18% SDS-polyacrylamide gel, transferred to nitrocellulose, and detected with the αHSP90C antibody (left gel). Alternatively, 2 μg of heterologously expressed HSP90C were separated on a 6% to 15% BN gel and visualized by silver staining (right gel).