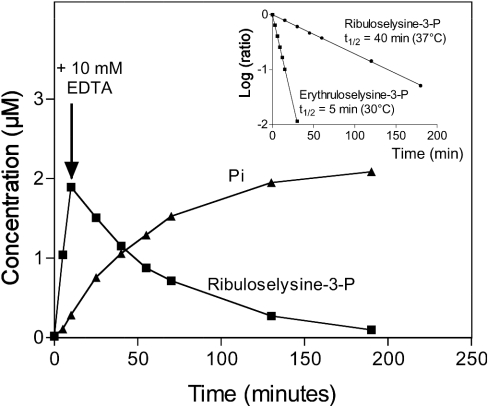
Figure 5. Stability of the putative ribuloselysine 3-phosphate and erythruloselysine 3-phosphate.
Ribulose-lysine was phosphorylated with the spinach leaf enzyme and [γ-32P]ATP. After 10 min, the reaction was stopped by adding 10 mM EDTA and the incubation was prolonged at 37 °C for the indicated times. The products (ribuloselysine 3-phosphate and inorganic phosphate) were analysed by anion-exchange chromatography. The same experiment was performed with erythruloselysine, but at 30 °C and using shorter incubation times. The inset shows the results expressed as the decimal logarithm of the ratio of the concentration of ketoamine-phosphates at the indicated times on their concentration when EDTA was added.