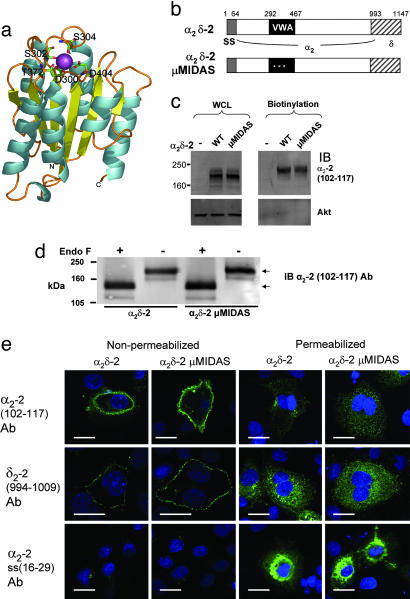
Fig. 1.
Mutation of the MIDAS in α2δ-2 does not prevent its trafficking to the plasma membrane. (a) Model of the α2δ-2 VWA domain. Residues comprising the MIDAS motif are labeled and shown as stick representations. The Mg2+ ion (purple) is coordinated within the MIDAS and is partially exposed at the surface in an appropriate manner for interaction with a second protein. α-helices are colored in cyan and β-strands in yellow. (b) The main domains in the primary sequence of α2δ-2, showing the extent of the VWA domain (black), and the three MIDAS mutations (white asterisks). SS, signal sequence. The amino acids making up the δ subunit are indicated by hatched boxes. (c) Cos-7 cells were transfected with either α2δ-2 or α2δ-2 μMIDAS, as indicated, and cell-surface proteins were biotinylated. (Left) Total expression in whole-cell lysate (WCL). (Right) Pull-down of biotinylated proteins on a 3–8% Tris acetate gel. (Upper) α2-2 (residues 102–117) Ab was used for immunoblotting (IB). (Lower) Anti-Akt Ab was used for IB to show that no intracellular proteins were biotinylated. The percentages of α2δ-2 and α2δ-2 μMIDAS at the cell surface were 10.0% and 9.5%, respectively (representative of three independent experiments; see Fig. 9 legend for calculation details). (d) Biotinylated α2δ-2 (Left) or α2δ-2 μMIDAS (Right), as in c, were treated (+) or not (–) with endoglycosidase F (5 units) and separated on a 7% Tris acetate gel. The upper arrow indicates fully glycosylated α2δ-2, and the lower arrow indicates deglycosylated α2δ-2. (e) Localization of α2δ-2 and α2δ-2 μMIDAS 48 h after transfection in Cos-7 cells that were either nonpermeabilized (Left) or permeabilized (Right) before immunostaining with the primary Abs shown. (Top) α2-2 (residues 102–117) Ab. (Middle) δ2-2(residues 994-1009)Ab. (Bottom) Signal peptide α2-2 (residues 16 –29) Ab. (Scale bar, 20 μm.)