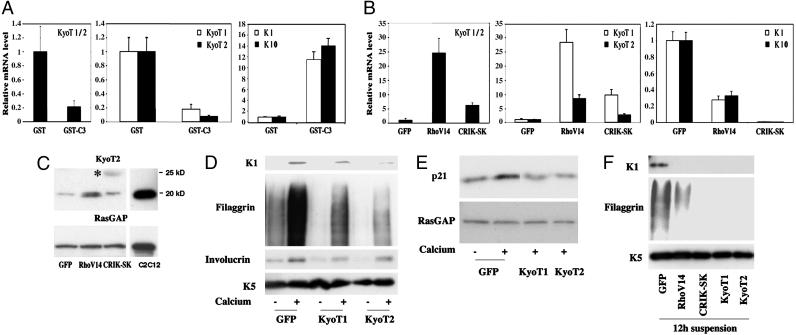
Fig. 5.
KyoT1/2 gene expression is up-regulated by the Rho/CRIK pathway and suppresses differentiation. (A) Keratinocytes were treated with the GST-C3 fusion protein or GST control for 9 h, and total RNA was analyzed by real-time RT-PCR with primers that recognize a common region of the KyoT1/2 transcript, as well as with primers specific for either one of these forms. The same RNAs were also analyzed by real-time RT-PCR for levels of K10 and K1 expression. (B) Keratinocytes were infected with the RhoV14, CRIK-SK, and GFP adenoviruses for 48 h, and total RNA was analyzed for levels of KyoT1 and KyoT2 as in the previous panel. To evaluate effects on K1 and K10 expression, a similar parallel analysis was performed on keratinocytes induced to differentiate by 9 h of calcium treatment. (C) Keratinocytes were infected with the RhoV14, CRIK-SK, and GFP adenoviruses, and total cell extracts were analyzed, in parallel with extracts from C2C12 myoblasts, by immunoblotting with antibodies against the KyoT2 protein. Immunoblotting for RasGAP was used as equal loading control. The larger size protein recognized by the anti-KyoT2 antibodies in the CRIK-SK expressing cells is indicated by an asterisk. (D and E) Mouse primary keratinocytes were infected with recombinant adenoviruses expressing the KyoT1 or KyoT2 proteins or GFP control for 48 h, and either kept under growing conditions or induced to differentiate by calcium for the last 10 h of the experiment. Total cell extracts were analyzed by immunoblotting with antibodies against the indicated differentiation markers (D) or against p21WAF1/Cip1 and RasGAP as equal loading control (E). (F) Primary keratinocytes were infected with the same adenoviruses as in the previous experiments in parallel with Ad-RhoV14 and Ad-CRIK-SK. Twenty-four hours after infection, cells were trypsinized and induced to differentiate by culture in suspension for another 12 h. Total cell extracts were analyzed by immunoblotting.