Abstract
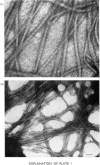
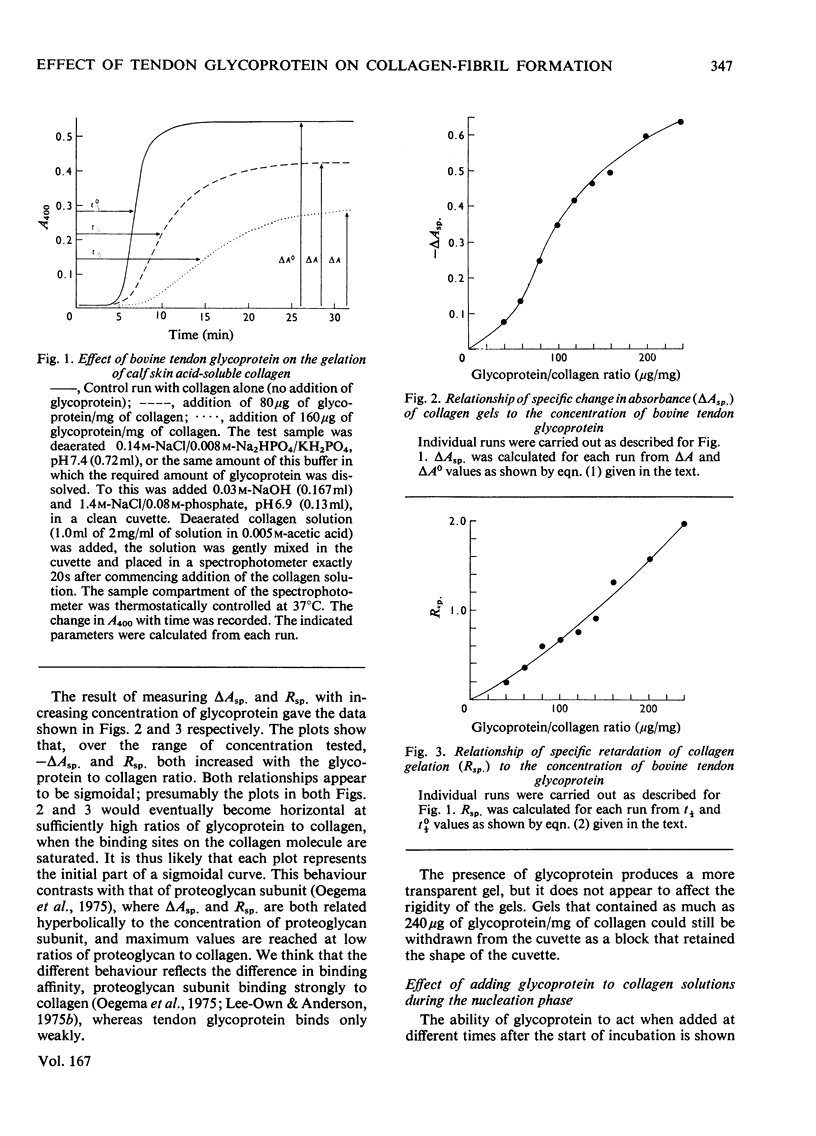
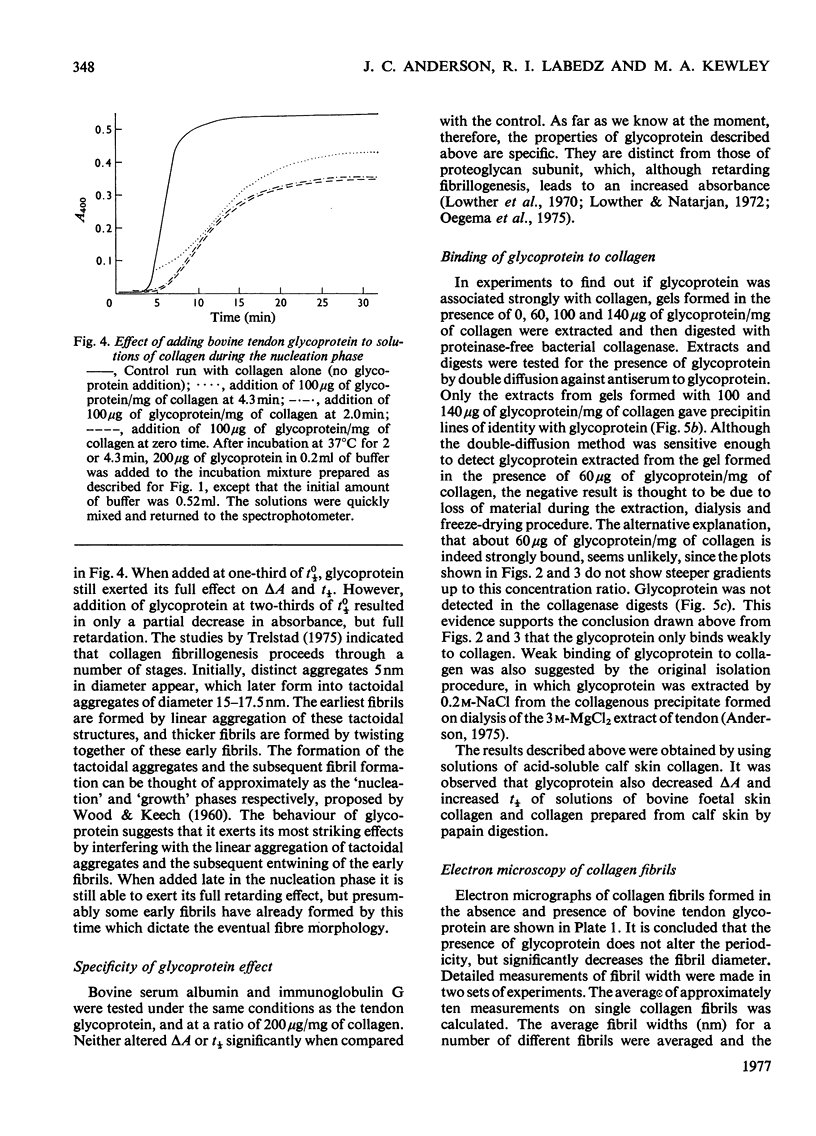
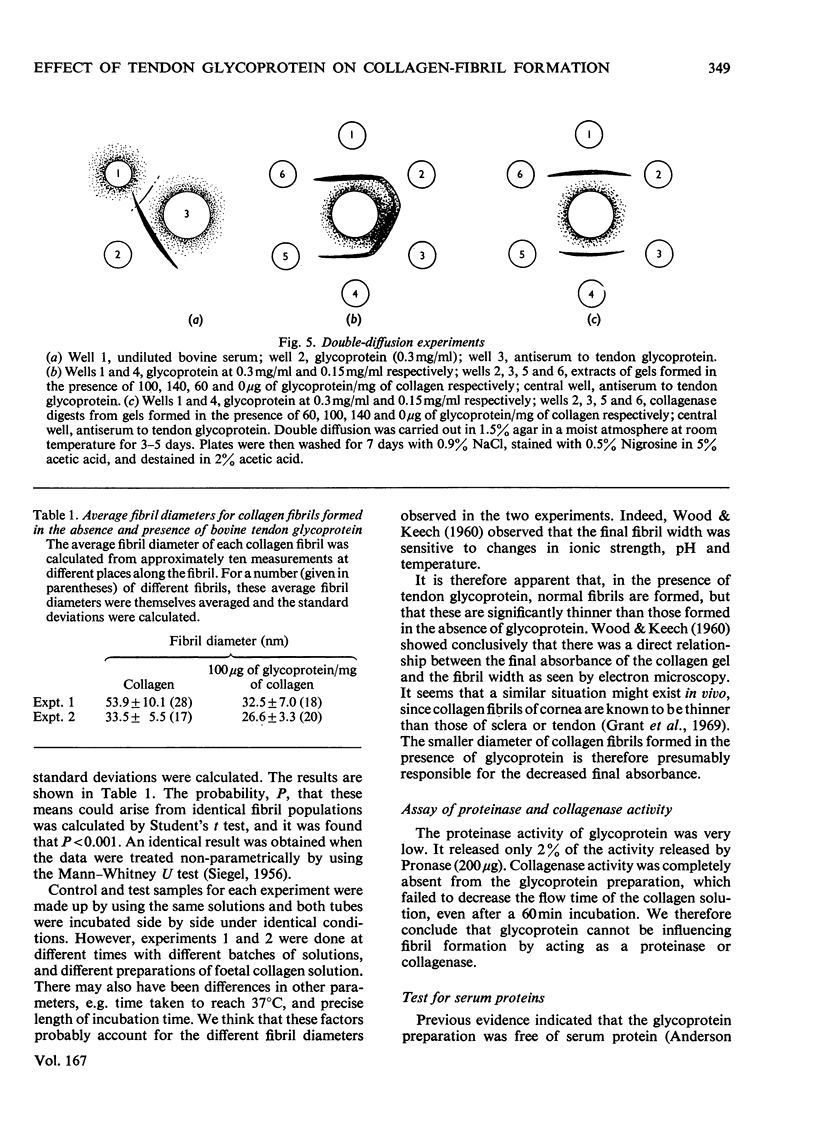
The formation of collagen fibrils under physiological conditions of ionic strength, pH and temperature was markedly affected by the presence of small amounts of bovine tendon glycoprotein. The absorbance of the gels at 400 nm was decreased, and they took longer to form. Over the range of concentration tested, the negative specific absorbance, -delta Asp., and the specific retardation, Rsp., both increased with the glycoprotein to collagen ratio. When added during the nucleation phase, glycoprotein was still able to exert its effect almost fully, and so must act to inhibit the later stages of fibril formation. Several pieces of evidence showed that glycoprotein acts via a weak binding to the collagen molecule. Electron microscopy established that fibrils formed in the presence of glycoprotein had a normal cross-striation pattern, but were significantly thinner than fibrils formed in control gets. The results suggest that glycoprotein could act in tissues to help regulate the diameter of collagen fibrils.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON A. J. THE FORMATION OF CHONDROMUCOPROTEIN-FIBRINOGEN AND CHONDROMUCOPROTEIN-BETA-LIPOPROTEIN COMPLEXES. Biochem J. 1963 Sep;88:460–469. doi: 10.1042/bj0880460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. C. Glycoproteins of the connective tissue matrix. Int Rev Connect Tissue Res. 1976;7:251–322. doi: 10.1016/b978-0-12-363707-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Anderson J. C. Isolation of a glycoprotein and proteodermatan sulphate from bovine achilles tendon by affinity chromatography on concanavalin A-Sepharose. Biochim Biophys Acta. 1975 Feb 27;379(2):444–455. doi: 10.1016/0005-2795(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Anderson J. C., Jackson D. S. The isolation of glycoproteins from bovine achilles tendon and their interaction with collagen. Biochem J. 1972 Mar;127(1):179–186. doi: 10.1042/bj1270179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. C., Labedz R. I., Brenchley P. E. Connective-tissue glycoconjugates of bovine tendon and skin. Biochem Soc Trans. 1977;5(2):431–433. doi: 10.1042/bst0050431. [DOI] [PubMed] [Google Scholar]
- Anderson J. C., Labedz R. I. Extractability of connective-tissue glycoprotein from bovine tendon. Biochem Soc Trans. 1977;5(2):434–435. doi: 10.1042/bst0050434. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Schmid K., Faris B., Beldekas J., Garvin P., Kagan H. M., Baum B. J. The interaction of collagen with alpha1-acid glycoprotein. Biochim Biophys Acta. 1976 Mar 18;427(1):302–314. doi: 10.1016/0005-2795(76)90306-8. [DOI] [PubMed] [Google Scholar]
- GALLOP P. M., SEIFTER S., MEILMAN E. Studies on collagen. I. The partial purification, assay, and mode of activation of bacterial collagenase. J Biol Chem. 1957 Aug;227(2):891–906. [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Grant M. E., Freeman I. L., Schofield J. D., Jackson D. S. Variations in the carbohydrate content of human and bovine polymeric collagens from various tissues. Biochim Biophys Acta. 1969 May 6;177(3):682–685. doi: 10.1016/0304-4165(69)90345-6. [DOI] [PubMed] [Google Scholar]
- HIGHBERGER J. H., GROSS J., SCHMITT F. O. The interaction of mucoprotein with soluble collagen; an electron microscope study. Proc Natl Acad Sci U S A. 1951 May;37(5):286–291. doi: 10.1073/pnas.37.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. Interaction between proteoglycan subunit and type II collagen from bovine nasal cartilage, and the preferential binding of proteoglycan subunit to type I collagen. Biochem J. 1976 Feb 1;153(2):259–264. doi: 10.1042/bj1530259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. The isolation of collagen-associated proteoglycan from bovine nasal cartilage and its preferential interaction with alpha2 chains of type I collagen. Biochem J. 1975 Jul;149(1):57–63. doi: 10.1042/bj1490057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. The preparation of a bacterial collagenase containing negligible non-specific protease activity. Prep Biochem. 1975;5(3):229–245. doi: 10.1080/00327487508061574. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Natarajan M. The influence of glycoprotein on collagen fibril formation in the presence of chondroitin sulphate proteoglycan. Biochem J. 1972 Apr;127(3):607–608. doi: 10.1042/bj1270607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Laidlaw J., Hascall V. C., Dziewiatkowski D. D. The effect of proteoglycans on the formation of fibrils from collagen solutions. Arch Biochem Biophys. 1975 Oct;170(2):698–709. doi: 10.1016/0003-9861(75)90167-8. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rajamäki A., Kulonen E. Purification on a structural glycoprotein from collagenase-digested experimental granuloma. Biochim Biophys Acta. 1971 Sep 28;243(3):398–406. doi: 10.1016/0005-2795(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S. Purification and amino acid composition of monomeric and polymeric collagens. Biochem J. 1967 Aug;104(2):534–536. doi: 10.1042/bj1040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C., KEECH M. K. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: kinetic and electron-microscope studies. Biochem J. 1960 Jun;75:588–598. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]