Abstract
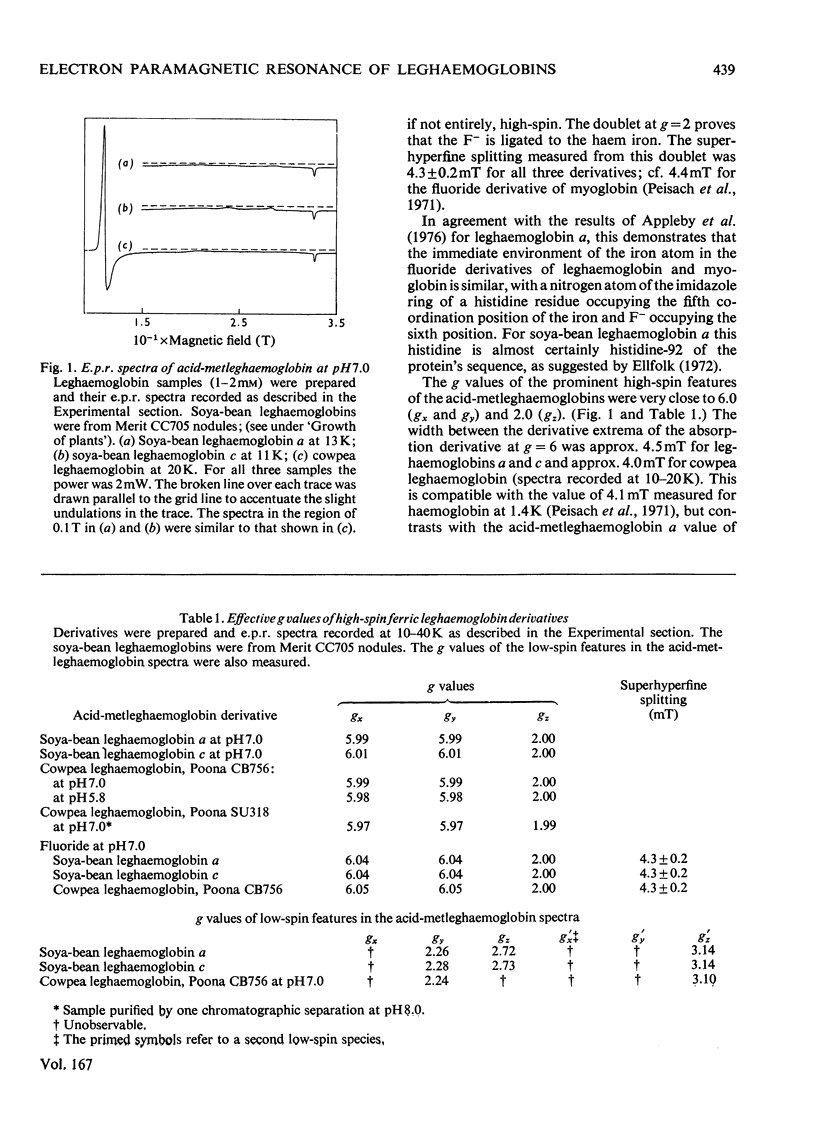
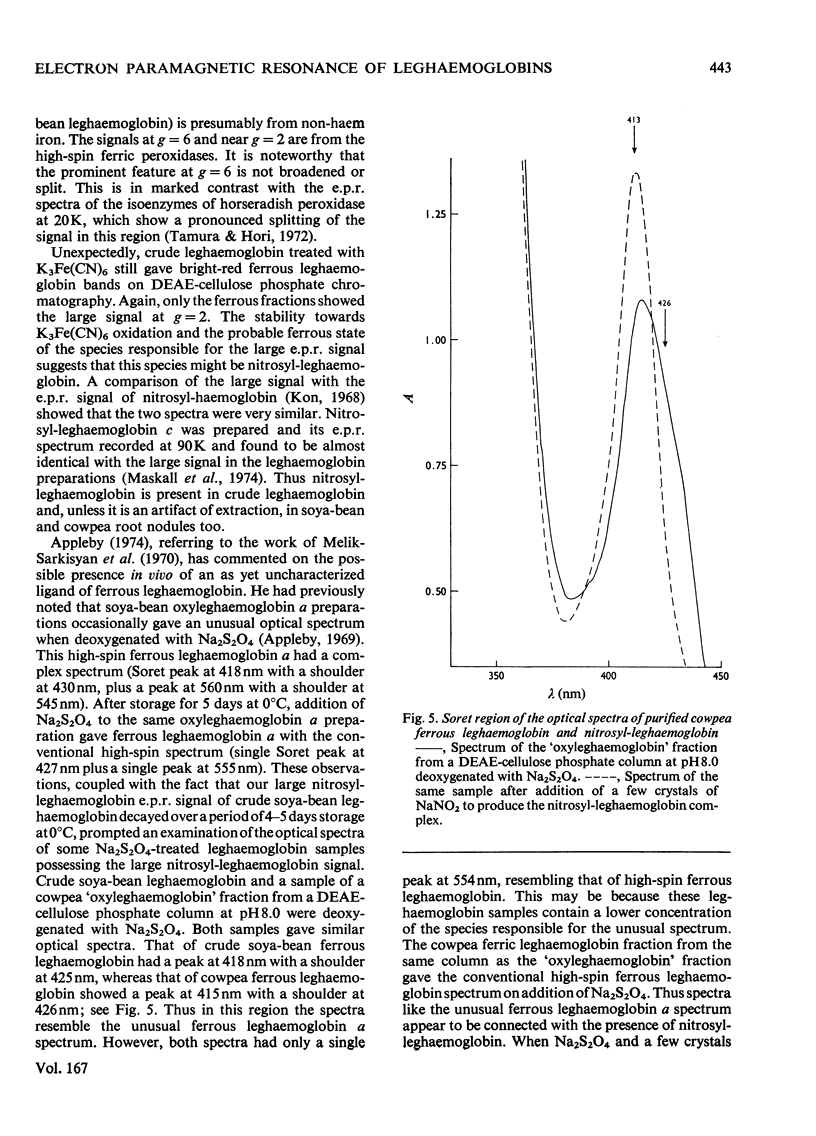
1. Leghaemoglobins from soya-bean (Glycine max) and cowpea (Vigna unguiculata) root nodules were purified by chromatography on DEAE-cellulose phosphate columns at pH8.0 and pH5.8, to avoid the relatively low pH (5.2) commonly used to purify these proteins. 2. E.p.r. (electron-paramagnetic-resonance) spectra of the fluoride, azide, hydroxide and cyanide complexes of these ferric leghaemoglobins were very similar to the spectra of the corresponding myoglobin derivatives, indicating that the immediate environment of the iron in leghaemoglobin and myoglobin is similar, an imidazole moiety of histidine being the proximal ligand to the haem iron [cf. Appleby, Blumberg, Peisach, Wittenberg & Wittenberg (1976) J. Biol. Chem. 251, 6090–6096]. 3. E.p.r. spectra of the acid-metleghaemoglobins showed prominent high-spin features very near g=6 and g=2 and, unlike myoglobin, small low-spin absorptions near g=2.26, 2.72 and 3.14. The width of the g=6 absorption derivative at 10–20K was about 4–4.5mT, similar to the value for acid-methaemoglobin. In contrast, a recently published (Appleby et al., 1976) spectrum of acid-metleghaemoglobin a had less high-spin character and a much broader absorption derivative around g=6. 4. E.p.r. spectra of ferric leghaemoglobin nicotinate and imidazole complexes suggest that the low-spin absorption near g=3.14 can be attributed to a trace of ferric leghaemoglobin nicotinate, and those near g=2.26 and 2.72 are from an endogenous dihistidyl haemichrome. 5. A large e.p.r. signal at g=2 in all samples of crude leghaemoglobin was shown to be from nitrosyl-leghaemoglobin. A soya-bean sample contained 27±3% of the latter. A previously unidentified form of soya-bean ferrous leghaemoglobin a was shown to be its nitrosyl derivative. If this is not an artifact, and occurs in the root nodule, the nitrosyl radical may interfere with the function of leghaemoglobin.
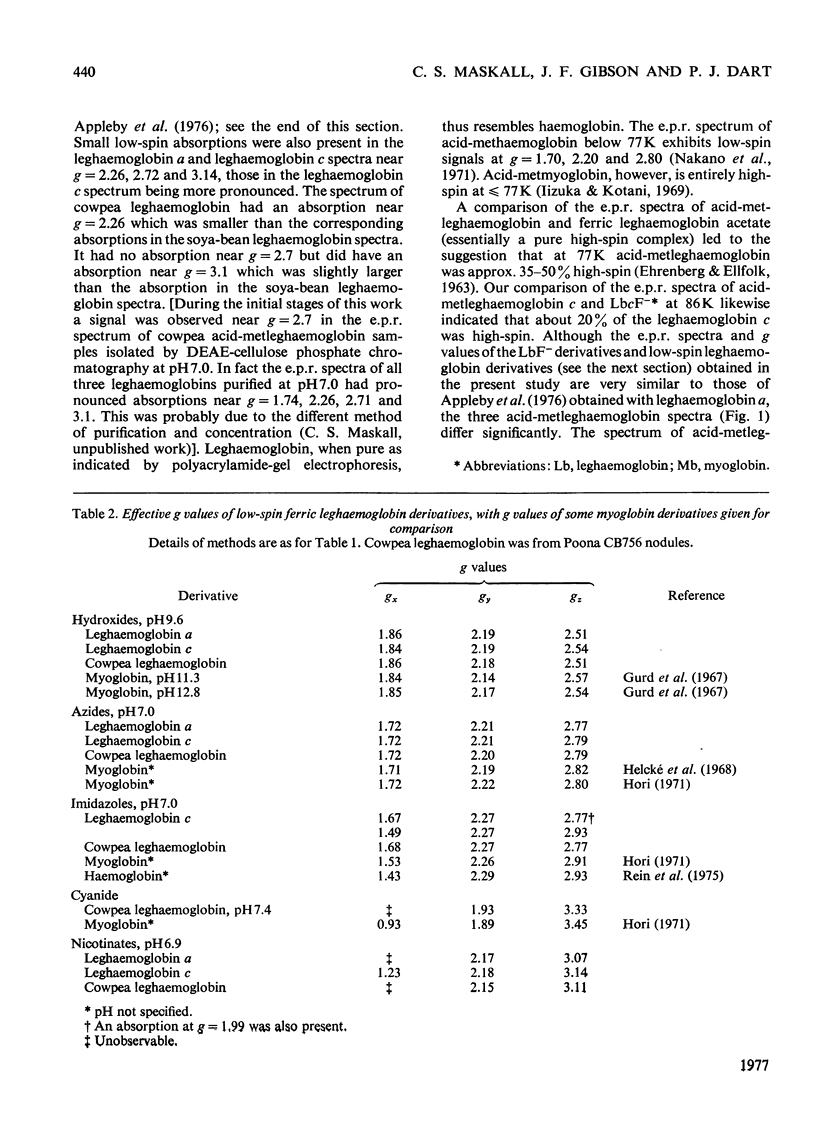
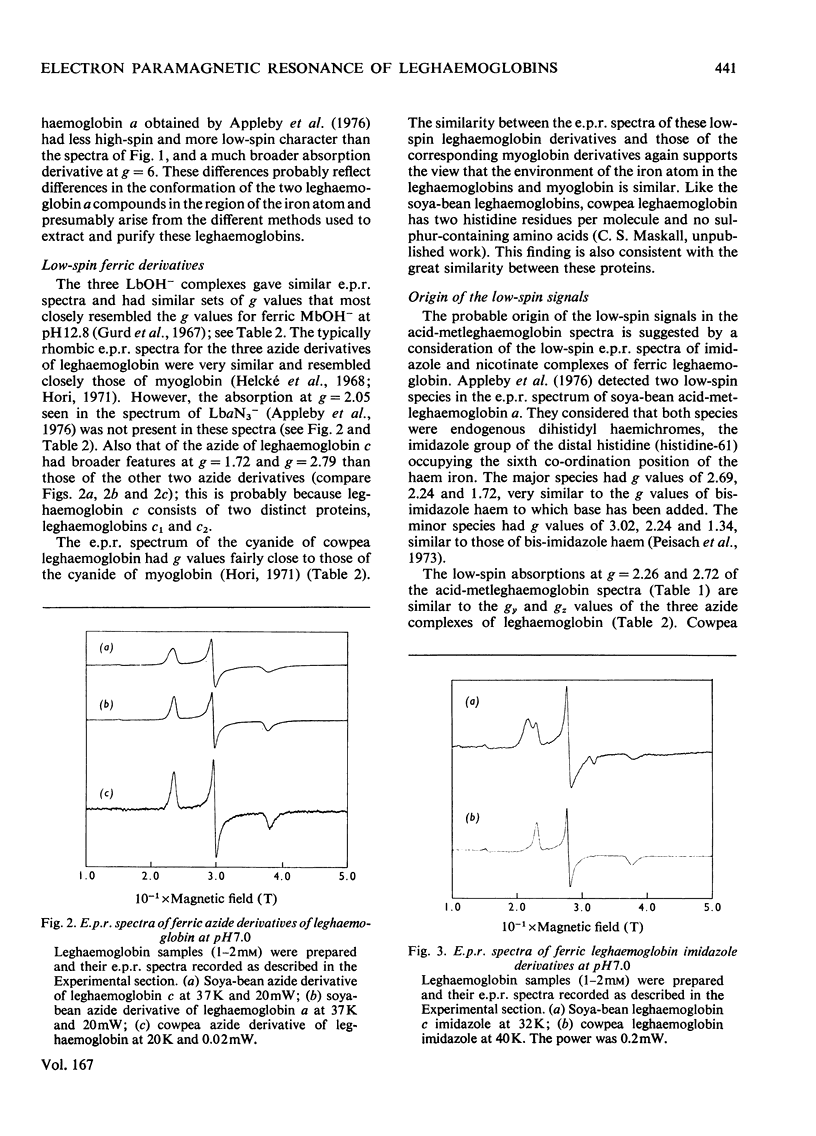
Full text
PDF





Selected References
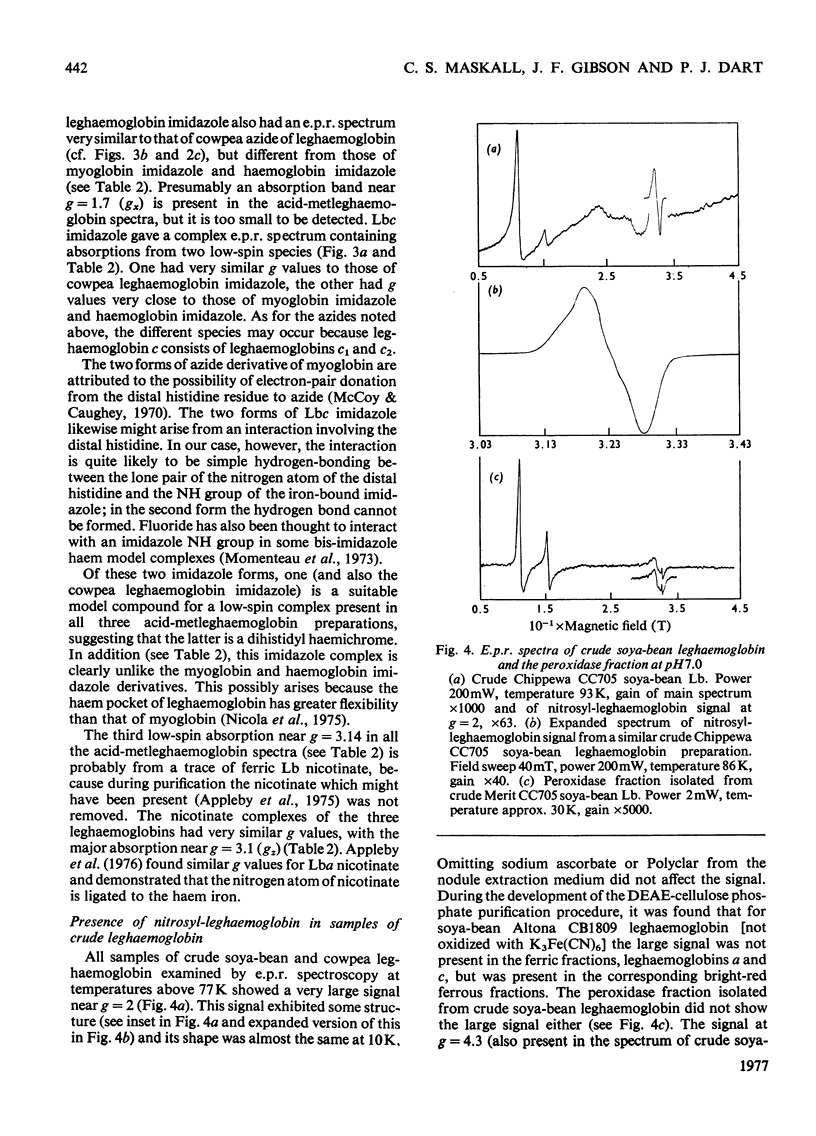
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Blumberg W. E., Peisach J., Wittenberg B. A., Wittenberg J. B. Leghemoglobin. An electron paramagnetic resonance and optical spectral study of the free protein and its complexes with nicotinate and acetate. J Biol Chem. 1976 Oct 10;251(19):6090–6096. [PubMed] [Google Scholar]
- Appleby C. A., Nicola N. A., Hurrell J. G., Leach S. J. Characterization and improved separation of soybean leghemoglobins. Biochemistry. 1975 Oct 7;14(20):4444–4450. doi: 10.1021/bi00691a016. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. The separation and properties of low-spin (haemochrome) and native, high-spin forms of leghaemoglobin from soybean nodule extracts. Biochim Biophys Acta. 1969 Oct 21;189(2):267–279. doi: 10.1016/0005-2728(69)90053-x. [DOI] [PubMed] [Google Scholar]
- Behlke J., Sievers G., Ellfolk N. Crystalline leghemoglobin. 13. Sedimentation studies. Acta Chem Scand. 1971;25(2):746–747. doi: 10.3891/acta.chem.scand.25-0746. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., McIntosh R. Reduction of methaemoglobin in haemoglobin samples using gel filtration for continuous removal of reaction products. Nature. 1967 Jan 28;213(5074):399–400. doi: 10.1038/213399a0. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Sievers G., Harmoinen A. Crystalline leghemoglobin. XV. Effect of urea on the conformation of the slow component (Lba). Acta Chem Scand B. 1974;28(10):1195–1199. doi: 10.3891/acta.chem.scand.28b-1195. [DOI] [PubMed] [Google Scholar]
- Geyer D., Lemberg R. The use of ferricyanide for the preparation of methaemoglobin. Biochim Biophys Acta. 1971 Jan 19;229(1):284–285. doi: 10.1016/0005-2795(71)90346-1. [DOI] [PubMed] [Google Scholar]
- Gurd F. R., Falk K. E., Malmström B. G., Vänngård T. A magnetic resonance study of sperm whale ferrimyoglobin and its complex with 1 cupric ion. J Biol Chem. 1967 Dec 25;242(24):5724–5730. [PubMed] [Google Scholar]
- Helcké G. A., Ingram D. J., Slade E. F. Electron resonance studies of haemoglobin derivatives. 3. Line-width and g-value measurements of acid-met myoglobin and of met myoglobin azide derivatives. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):275–288. doi: 10.1098/rspb.1968.0011. [DOI] [PubMed] [Google Scholar]
- Henry Y., Banerjee R. Electron paramagnetic studies of nitric oxide haemoglobin derivatives: isolated subunits and nitric oxide hybrids. J Mol Biol. 1973 Feb 5;73(4):469–482. doi: 10.1016/0022-2836(73)90094-6. [DOI] [PubMed] [Google Scholar]
- Hori H. Analysis of the principal g-tensors in single crystals of ferrimyoglobin complexes. Biochim Biophys Acta. 1971 Nov 19;251(2):227–235. doi: 10.1016/0005-2795(71)90106-1. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Kotani M. Analysis of thermal equilibrium between high-spin and low-spin states in ferrimyoglobin complexes. Biochim Biophys Acta. 1969 May;181(1):275–286. doi: 10.1016/0005-2795(69)90250-5. [DOI] [PubMed] [Google Scholar]
- Imamura T., Riggs A. Equilibria and kinetics of ligand binding by leghemoglobin from soybean root nodules. J Biol Chem. 1972 Jan 25;247(2):521–526. [PubMed] [Google Scholar]
- Jackson E. K., Evans H. J. Propionate in heme biosynthesis in soybean nodules. Plant Physiol. 1966 Dec;41(10):1673–1680. doi: 10.1104/pp.41.10.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon H. Paramagnetic resonance study of Nitric Oxide hemoglobin. J Biol Chem. 1968 Aug 25;243(16):4350–4357. [PubMed] [Google Scholar]
- McCoy S., Caughey W. Infrared studies of azido, cyano, and other derivatives of metmyoglobin, methemoglobin, and hemins. Biochemistry. 1970 Jun 9;9(12):2387–2393. doi: 10.1021/bi00814a001. [DOI] [PubMed] [Google Scholar]
- Melik-Sarkisian S. S., Iarovenko V. V., Kretovich V. L. O prirode legoglobina v kluben'kakh liupina. Biokhimiia. 1970 Nov-Dec;35(6):1230–1237. [PubMed] [Google Scholar]
- Miltimore J. E., Mason J. L., McArthur J. M., Carson R. B. Ruminant Mineral Nutrition. The Effect of Copper Injections on Weight Gains and Haemoglobin Levels of Cattle Pastured on Ground-Water Soils in The British Columbia Interior. Can J Comp Med Vet Sci. 1964 May;28(5):108–112. [PMC free article] [PubMed] [Google Scholar]
- Momenteau M., Mispelter J., Lexa D. The physical chemistry of hemes. II. Electron paramagnetic resonance study of the interaction of some iron(3)-porphyrins with fluoride ion in dimethylformamide. Biochim Biophys Acta. 1973 Oct 5;320(3):652–662. doi: 10.1016/0304-4165(73)90145-1. [DOI] [PubMed] [Google Scholar]
- Nakano N., Nakano K., Tasaki A. A magnetic study of acidic ferric hemoglobin. Biochim Biophys Acta. 1971 Dec 28;251(3):303–313. doi: 10.1016/0005-2795(71)90116-4. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Minasian E., Appleby C. A., Leach S. J. Circular dichroism studies of myoglobin and leghemoglobin. Biochemistry. 1975 Nov 18;14(23):5141–5149. doi: 10.1021/bi00694a019. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Adler A. Electron paramagnetic resonance studies of iron porphin and chlorin systems. Ann N Y Acad Sci. 1973;206:310–327. doi: 10.1111/j.1749-6632.1973.tb43219.x. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Ogawa S., Rachmilewitz E. A., Oltzik R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J Biol Chem. 1971 May 25;246(10):3342–3355. [PubMed] [Google Scholar]
- Rein H., Ristau O., Ruckpaul K. Evidence for the existence of a low spin complex in acidic methemoglobin: its structure and formation. Biochim Biophys Acta. 1975 Jun 26;393(2):373–378. doi: 10.1016/0005-2795(75)90064-1. [DOI] [PubMed] [Google Scholar]
- Seevers P. M., Daly J. M., Catedral F. F. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 1971 Sep;48(3):353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Hori H. Optical and magnetic measurements of horseradish peroxidase. 3. Electron paramagnetic resonance studies at liquid-hydrogen and -helium temperatures. Biochim Biophys Acta. 1972 Sep 19;284(1):20–29. doi: 10.1016/0005-2744(72)90041-1. [DOI] [PubMed] [Google Scholar]
- Vainshtein B. K., Harutyunyan E. H., Kuranova I. P., Borisov V. V., Sosfenov N. I., Pavlovsky A. G., Grebenko A. I., Konareva N. V. Structure of leghaemoglobin from lupin root nodules at 5 angstrom resolution. Nature. 1975 Mar 13;254(5496):163–164. doi: 10.1038/254163a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr, Reed G. H. Electromagnetic properties of hemoproteins. V. Optical and electron paramagnetic resonance characteristics of nitric oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972 Apr 25;247(8):2447–2455. [PubMed] [Google Scholar]


