Abstract
Investigation of aldolase 1, the class-I D-fructose 1,6-bisphosphate aldolase (EC4.1.2.13) from Escherichia coli (Crookes' strain), showed it to have unusual kinetic and structural properties. The enzyme appeared to be larger than was previously supposed and may be a decamer with a mol. wt. of approx. 340000. Its fructose 1,6-bisphosphate-cleavage activity was unaffected by these compounds. The enhancement exhibited a strong dependence on pH. These novel kinetic properties do not seem to be shared by any other fructose 1,6-bisphosphate aldolase, but recall the activation by polycarboxylic acids of the deoxyribose 3-phosphate aldolases from some other organisms. In view of its unusual properties, it is unlikely that aldolase 1 from E. coli is closely related to the class-1 aldolases that have been detected in several other prokaryotes, or to the typical class-1 enzymes from eukaryotes.
Full text
PDF


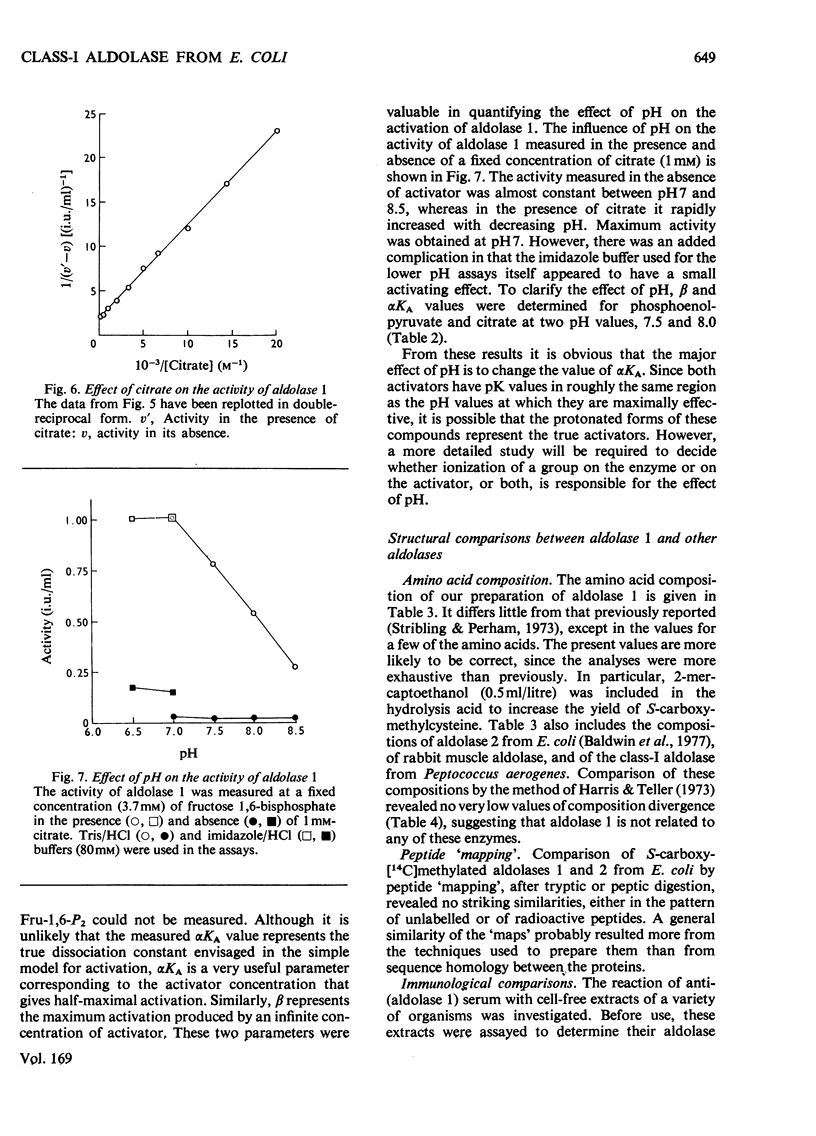
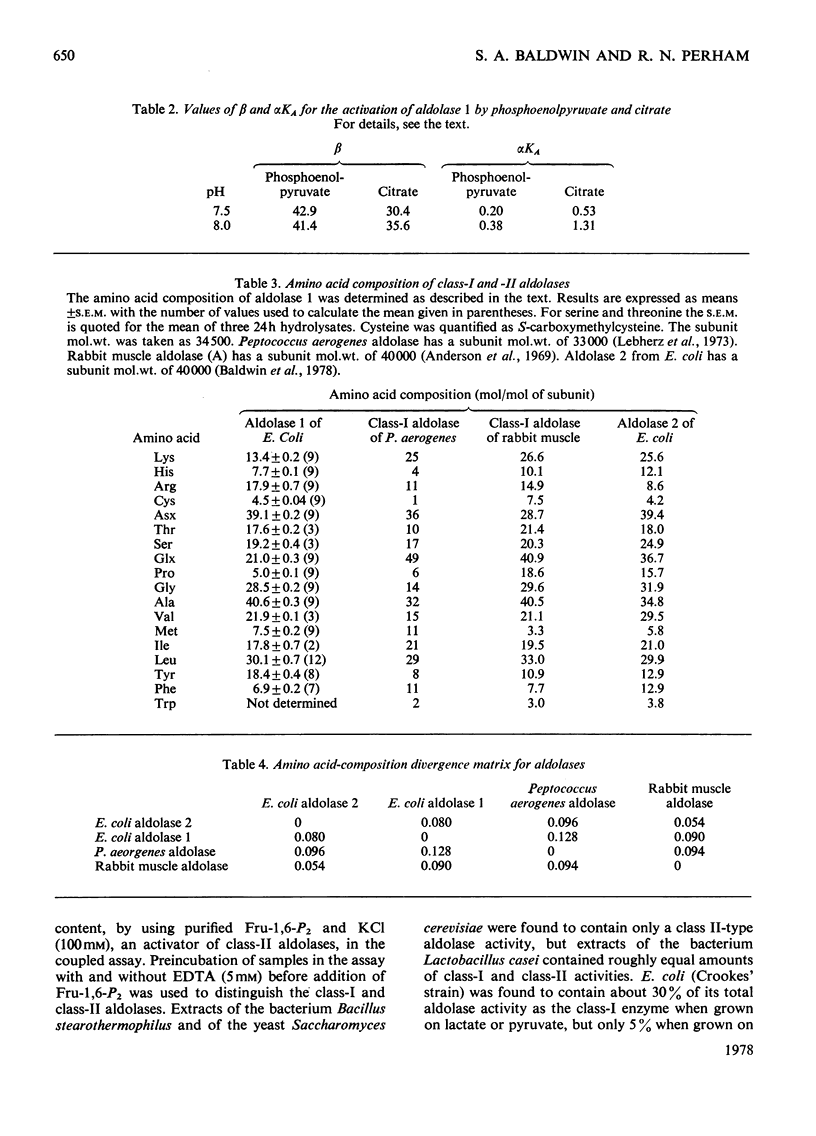


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Gibbons I., Perham R. N. A comparative study of the structure of muscle fructose 1,6-diphosphate aldolases. Eur J Biochem. 1969 Dec;11(3):503–509. doi: 10.1111/j.1432-1033.1969.tb00802.x. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Baldwin S. A., Perham R. N., Stribling D. Purification and characterization of the class-II D-fructose 1,6-bisphosphate aldolase from Escherichia coli (Crookes' strain). Biochem J. 1978 Mar 1;169(3):633–641. doi: 10.1042/bj1690633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Boeker E. A., Snell E. E. Arginine decarboxylase from Escherichia coli. II. Dissociation and reassociation of subunits. J Biol Chem. 1968 Apr 25;243(8):1678–1684. [PubMed] [Google Scholar]
- Groth D. P. Deoxyribose 5-phosphate aldolase. II. Purification and properties of the rat liver enzyme. J Biol Chem. 1967 Jan 10;242(1):155–159. [PubMed] [Google Scholar]
- Harris C. E., Teller D. C. Estimation of primary sequence homology from amino acid composition of evolutionary related proteins. J Theor Biol. 1973 Feb;38(2):347–362. doi: 10.1016/0022-5193(73)90179-3. [DOI] [PubMed] [Google Scholar]
- Hucho F., Müllner H., Sund H. Investigation of the symmetry of oligomeric enzymes with bifunctional reagents. Eur J Biochem. 1975 Nov 1;59(1):79–87. doi: 10.1111/j.1432-1033.1975.tb02427.x. [DOI] [PubMed] [Google Scholar]
- JIANG N. S., GROTH D. P. Polycarboxylic acid activation of rat liver deoxyribose phosphate aldolase. J Biol Chem. 1962 Nov;237:3339–3341. [PubMed] [Google Scholar]
- Jayanthi Bia N., Ramachandra Pai M., Suryanarayana Murthy P., Venkitasubramanian T. A. Fructose diphosphate aldolase from Mycobacterium smegmatis. Purification and properties. Arch Biochem Biophys. 1975 May;168(1):230–234. doi: 10.1016/0003-9861(75)90245-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebherz H. G., Bradshaw R. A., Rutter W. J. Structural comparisons between the class I fructose diphosphate aldolases from Micrococcus aerogenes and rabbit. J Biol Chem. 1973 Mar 10;248(5):1660–1665. [PubMed] [Google Scholar]
- Lebherz H. G., Rutter W. J. A class I (Schiff base) fructose diphosphate aldolase of prokaryotic origin. Purification and properties of Micrococcus aerogenes aldolase. J Biol Chem. 1973 Mar 10;248(5):1650–1659. [PubMed] [Google Scholar]
- London J. Variations in the quaternary structure of three lactic acid bacteria aldolases. Evidence for the existence of a class I and class II aldolase in Lactobacillus casei. J Biol Chem. 1974 Dec 25;249(24):7977–7983. [PubMed] [Google Scholar]
- Morse D. E., Horecker B. L. The mechanism of action of aldolases. Adv Enzymol Relat Areas Mol Biol. 1968;31:125–181. doi: 10.1002/9780470122761.ch4. [DOI] [PubMed] [Google Scholar]
- OSEN O. M., HOFFEE P., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. VII. FORMATION OF A 2-METHYL-2-DEOXYPENTOSE CATALYZED BY DEOXYRIBOSE 5-PHOSPHATE ALDOLASE. J Biol Chem. 1965 Apr;240:1517–1524. [PubMed] [Google Scholar]
- PRICER W. E., Jr, HORECKER B. L. Deoxyribose aldolase from Lactobacillus plantarum. J Biol Chem. 1960 May;235:1292–1298. [PubMed] [Google Scholar]
- Penhoet E. E., Kochman M., Rutter W. J. Molecular and catalytic properties of aldolase C. Biochemistry. 1969 Nov;8(11):4396–4402. doi: 10.1021/bi00839a026. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. The subunit molecular weights of the alpha-ketoacid dehydrogenase multienzyme complexes from E. coli. FEBS Lett. 1971 Jun 2;15(1):8–12. doi: 10.1016/0014-5793(71)80066-2. [DOI] [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Stribling D., Perham R. N. Purification and characterization of two fructose diphosphate aldolases from Escherichia coli (Crookes' strain). Biochem J. 1973 Apr;131(4):833–841. doi: 10.1042/bj1310833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WOLFF J. B., KAPLAN N. O. D-Mannitol 1-phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1956 Feb;218(2):849–869. [PubMed] [Google Scholar]
- Webster D., Jondorf W. R., Dixon H. B. Phosphonomethyl analogues of hexose phosphates. Biochem J. 1976 May 1;155(2):433–441. doi: 10.1042/bj1550433. [DOI] [PMC free article] [PubMed] [Google Scholar]