Figure 4.
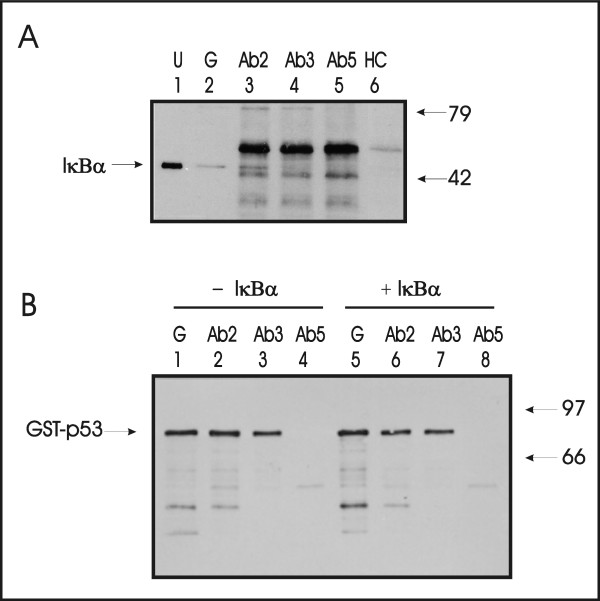
A monoclonal antibody recognizing an epitope in the DNA binding domain of p53 (Ab3) interferes with IκBα binding to p53. Purified p53 with a glutathione binding protein epitope tag (GST-p53) and purified IκBα protein were incubated together in vitro. A. P53 was precipitated either with glutathione Sepharose (denoted G, lane 2) or with p53-specific monoclonal antibodies Ab2 (lane 3), Ab3 (lane 4), or Ab5 (lane 5) and Sephadex protein A/G beads. Precipitated proteins were separated by SDS-PAGE and detected by Western blotting with a rabbit polyclonal antiserum directed against the N-terminus of IκBα. Mobility of IκBα protein is indicated (lane 1). This also represents the total input IκBα. Similar quantities of murine immunoglobulin heavy chain (HC, lane 6) were precipitated by protein A/G beads and served as the negative control. B. GST-p53 was quantitatively precipitated in the absence (- IκBα) or presence (+ IκBα) of IκBα by glutathione Sepharose (denoted G, lanes 1,5), Ab2 (lanes 2,6), and Ab3 (lanes 3,7), but not Ab5 (lanes 4,8), as detected with a rabbit polyclonal p53 antiserum. This blot is essentially identical to that shown in panel A but for the antibody used in the Western blotting step. Since Ab5 did not precipitate GST-p53, it served as a negative control for non-specific association between IκBα protein and either antibody or protein A/G beads.
