Abstract
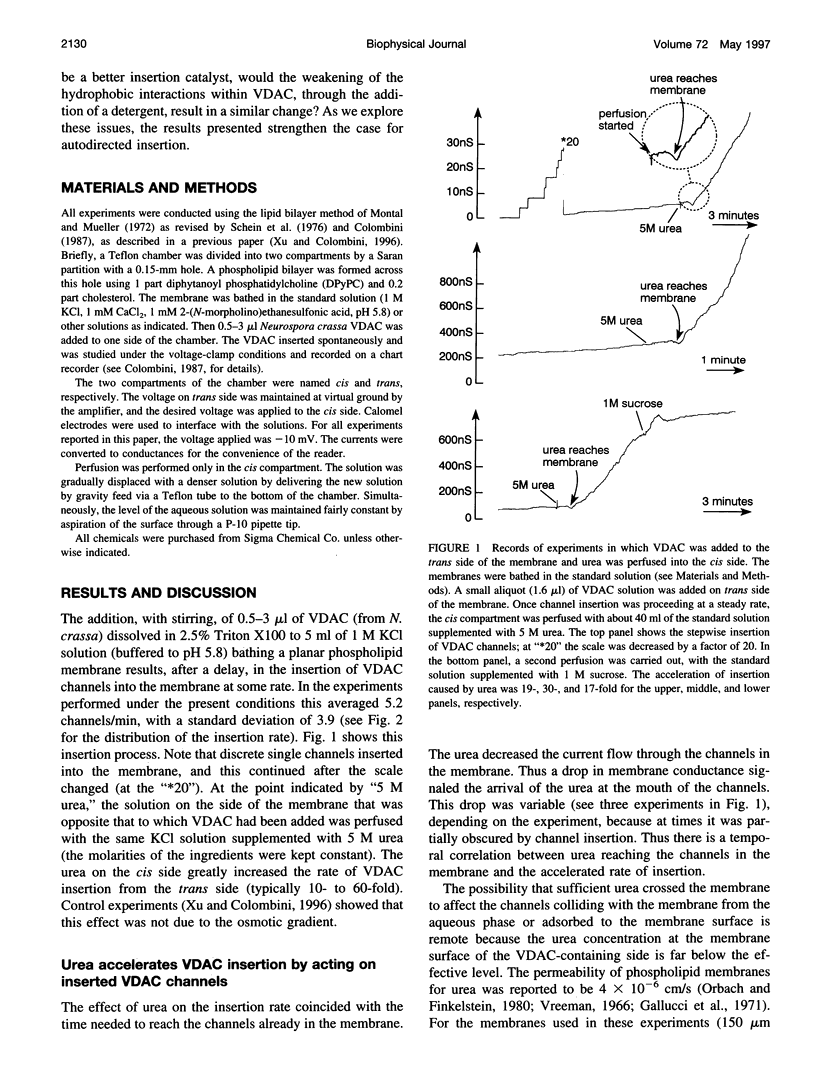
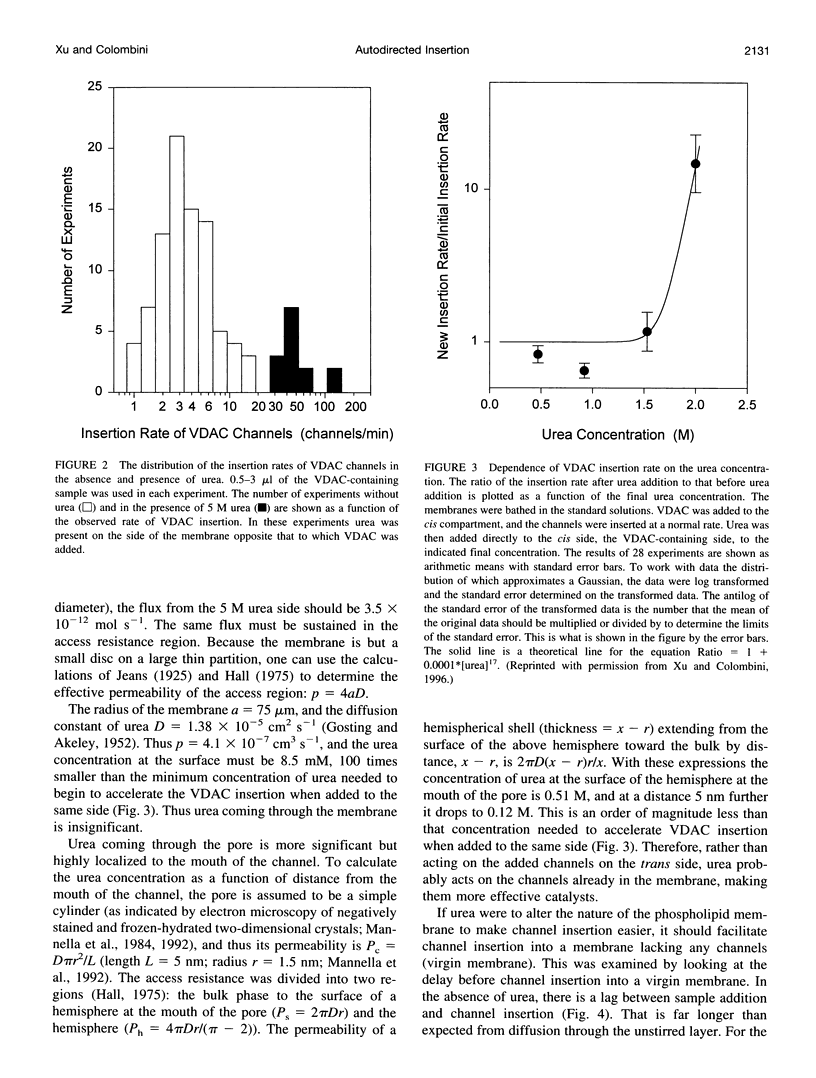
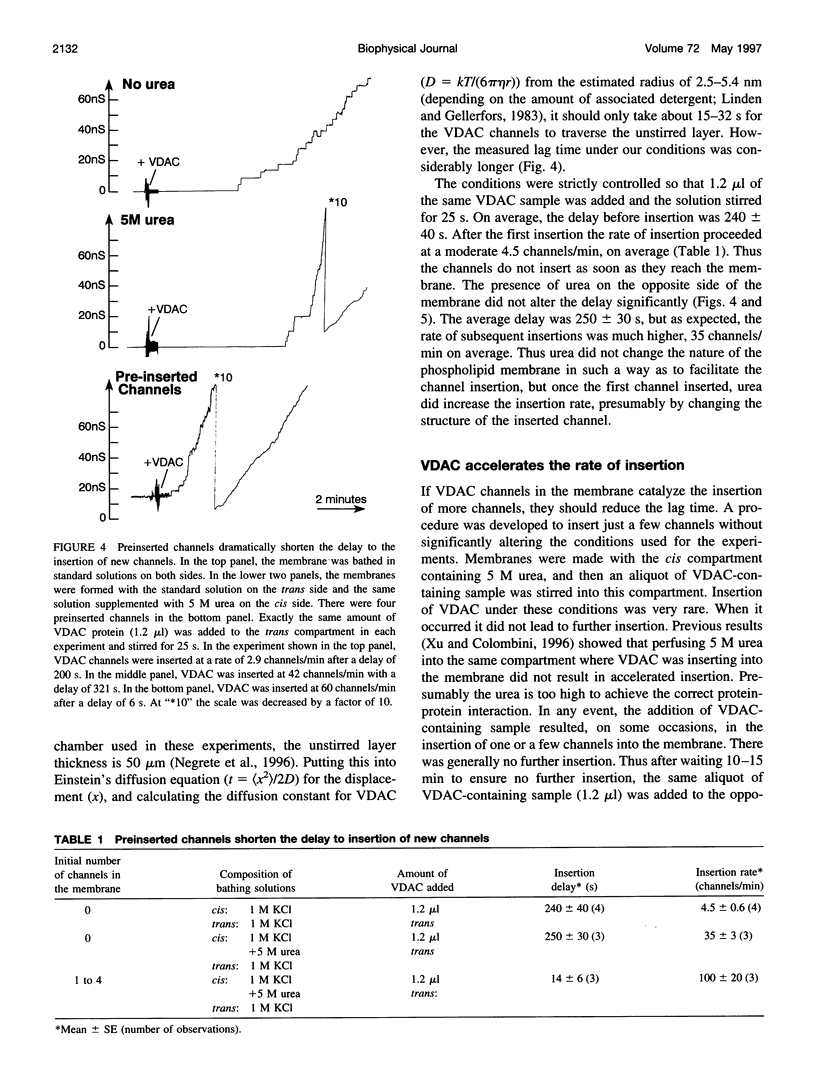
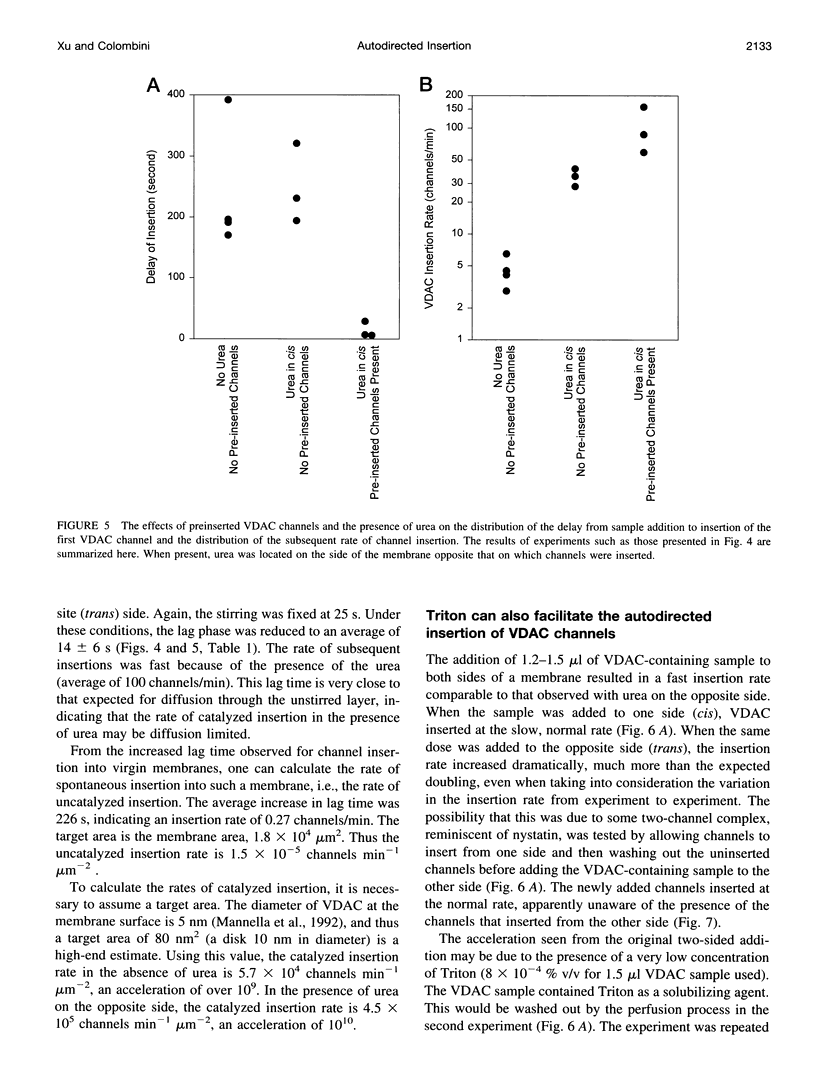
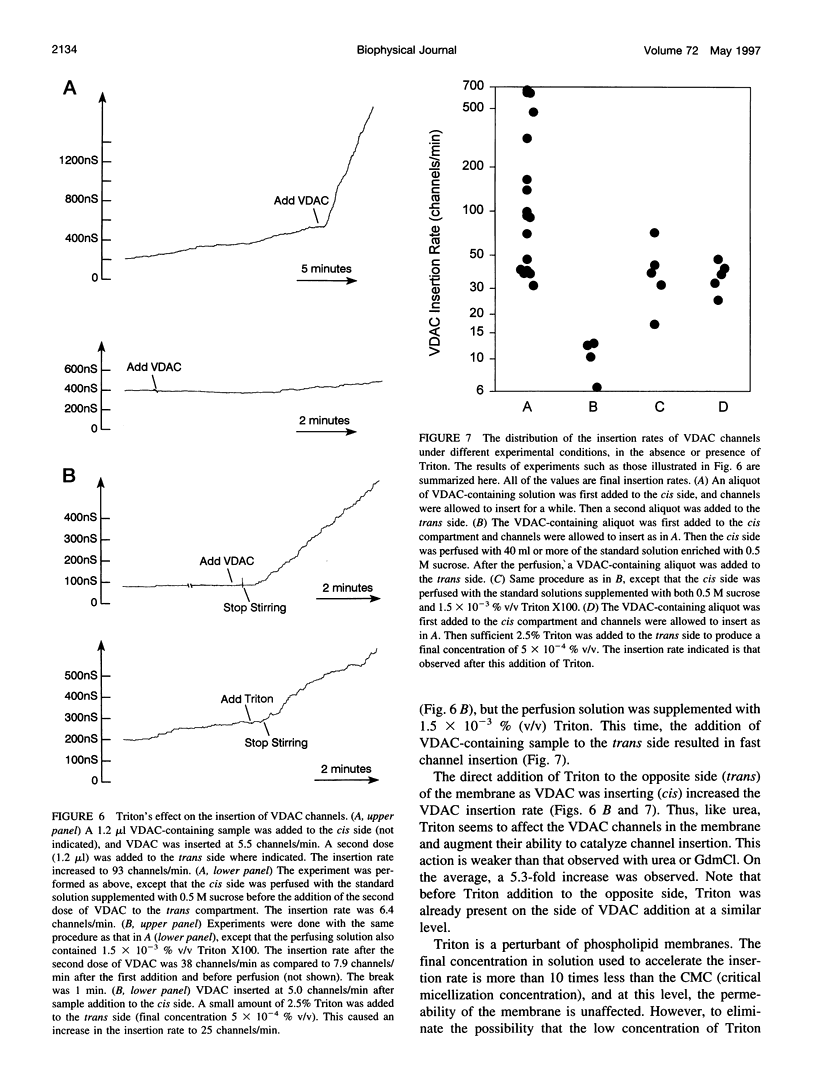
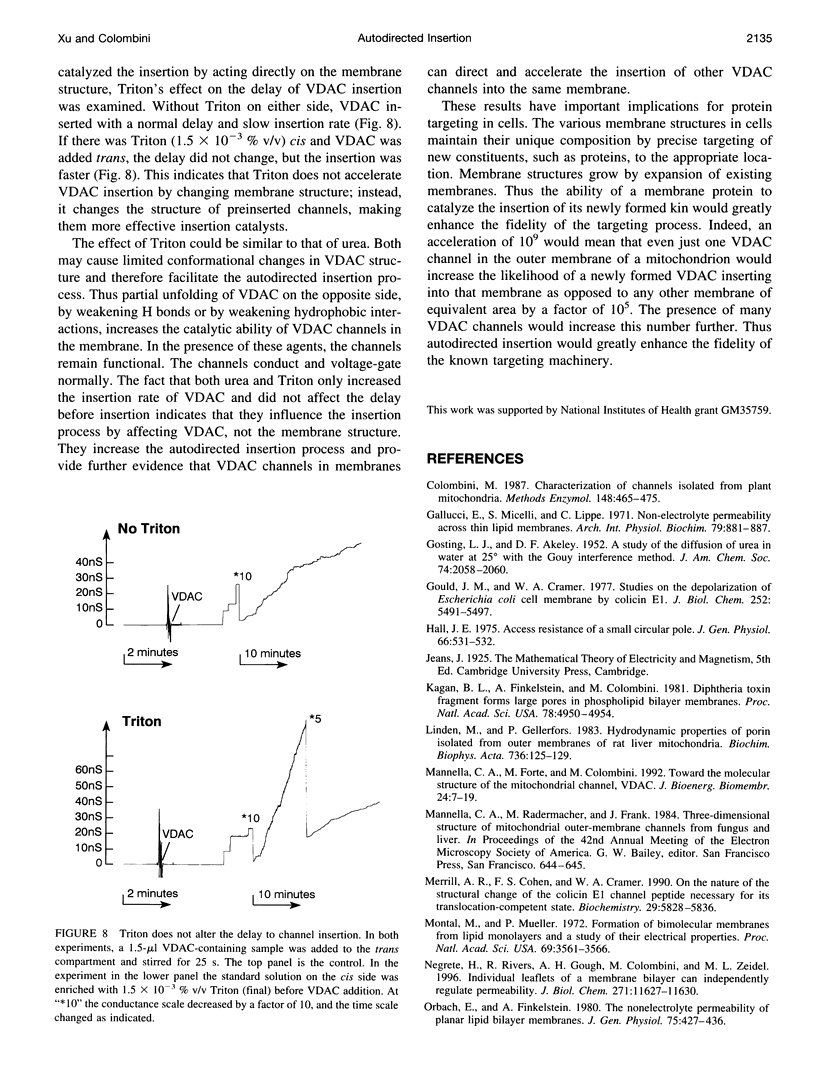
VDAC, a mitochondrial outer membrane channel, has the ability to catalyze and direct the insertion of other VDAC channels into planar phospholipid membranes. The spontaneous rate of insertion of detergent-solubilized VDAC channels into phospholipid membranes is estimated to be 1.5 x 10(-5) channels min-1 micron-2. VDAC channels already in the membrane can increase this rate by a factor of 10(9). The presence of 5 M urea on the opposite side of the membrane increases this 10-fold to 4.5 x 10(5) channels min-1 microns-2. Similar but weaker effects are observed with Triton X100 addition (10(-3)% (v/v)). These agents are not acting on uninserted channels because they do not affect the delay from sample addition to first insertion. Under the chosen conditions, this delay is long (240 s) without preinserted channels. However, the presence of a few VDAC channels in the membrane reduces this delay to 14 s, close to the diffusion limit. Therefore, urea and Triton, added to the side of the membrane opposite that to which the VDAC sample was added, likely increase the flexibility of the VDAC channels in the membrane, allowing them to be more efficient catalysts for VDAC insertion. There are obvious implications for membrane protein insertion and targeting.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colombini M. Characterization of channels isolated from plant mitochondria. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- Gallucci E., Micelli S., Lippe C. Non-electrolyte permeability across thin lipid membranes. Arch Int Physiol Biochim. 1971 Dec;79(5):881–887. doi: 10.3109/13813457109104847. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Hall J. E. Access resistance of a small circular pore. J Gen Physiol. 1975 Oct;66(4):531–532. doi: 10.1085/jgp.66.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P. Hydrodynamic properties of porin isolated from outer membranes of rat liver mitochondria. Biochim Biophys Acta. 1983 Dec 7;736(1):125–129. doi: 10.1016/0005-2736(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Forte M., Colombini M. Toward the molecular structure of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992 Feb;24(1):7–19. doi: 10.1007/BF00769525. [DOI] [PubMed] [Google Scholar]
- Merrill A. R., Cohen F. S., Cramer W. A. On the nature of the structural change of the colicin E1 channel peptide necessary for its translocation-competent state. Biochemistry. 1990 Jun 19;29(24):5829–5836. doi: 10.1021/bi00476a026. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete H. O., Rivers R. L., Goughs A. H., Colombini M., Zeidel M. L. Individual leaflets of a membrane bilayer can independently regulate permeability. J Biol Chem. 1996 May 17;271(20):11627–11630. doi: 10.1074/jbc.271.20.11627. [DOI] [PubMed] [Google Scholar]
- Orbach E., Finkelstein A. The nonelectrolyte permeability of planar lipid bilayer membranes. J Gen Physiol. 1980 Apr;75(4):427–436. doi: 10.1085/jgp.75.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Xu X., Colombini M. Self-catalyzed insertion of proteins into phospholipid membranes. J Biol Chem. 1996 Sep 27;271(39):23675–23682. doi: 10.1074/jbc.271.39.23675. [DOI] [PubMed] [Google Scholar]
- Zizi M., Thomas L., Blachly-Dyson E., Forte M., Colombini M. Oriented channel insertion reveals the motion of a transmembrane beta strand during voltage gating of VDAC. J Membr Biol. 1995 Mar;144(2):121–129. doi: 10.1007/BF00232798. [DOI] [PubMed] [Google Scholar]


