Abstract
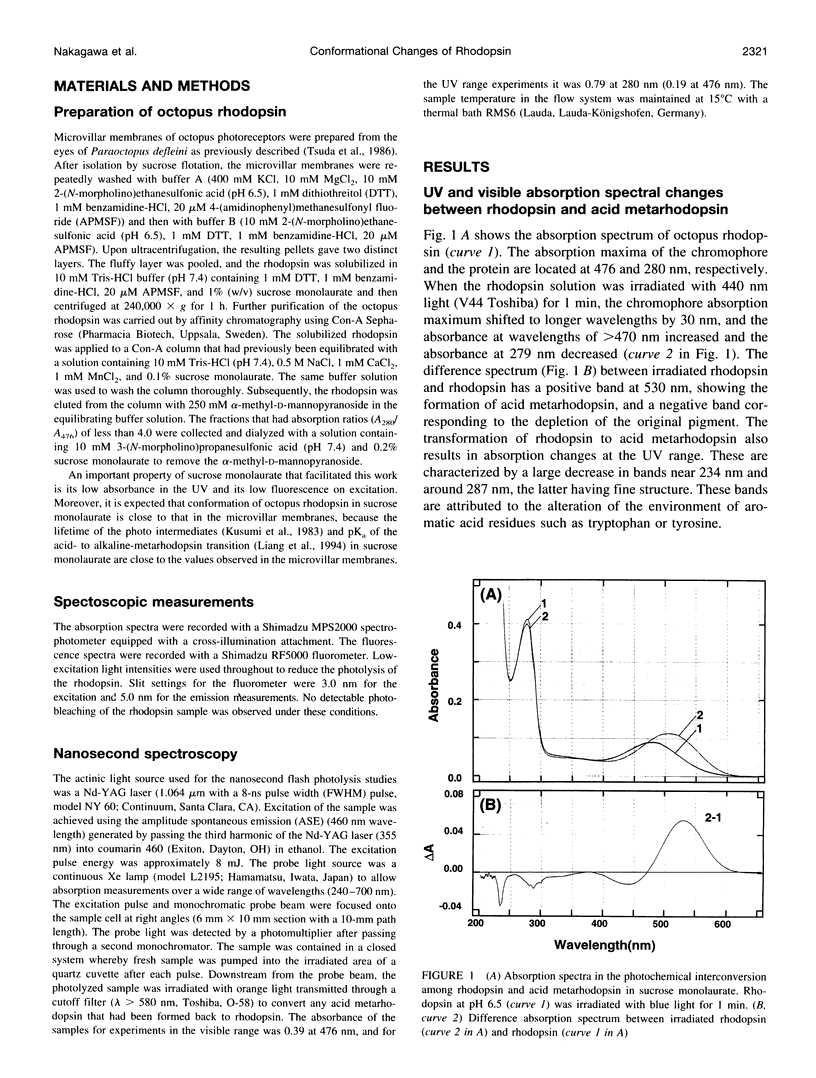
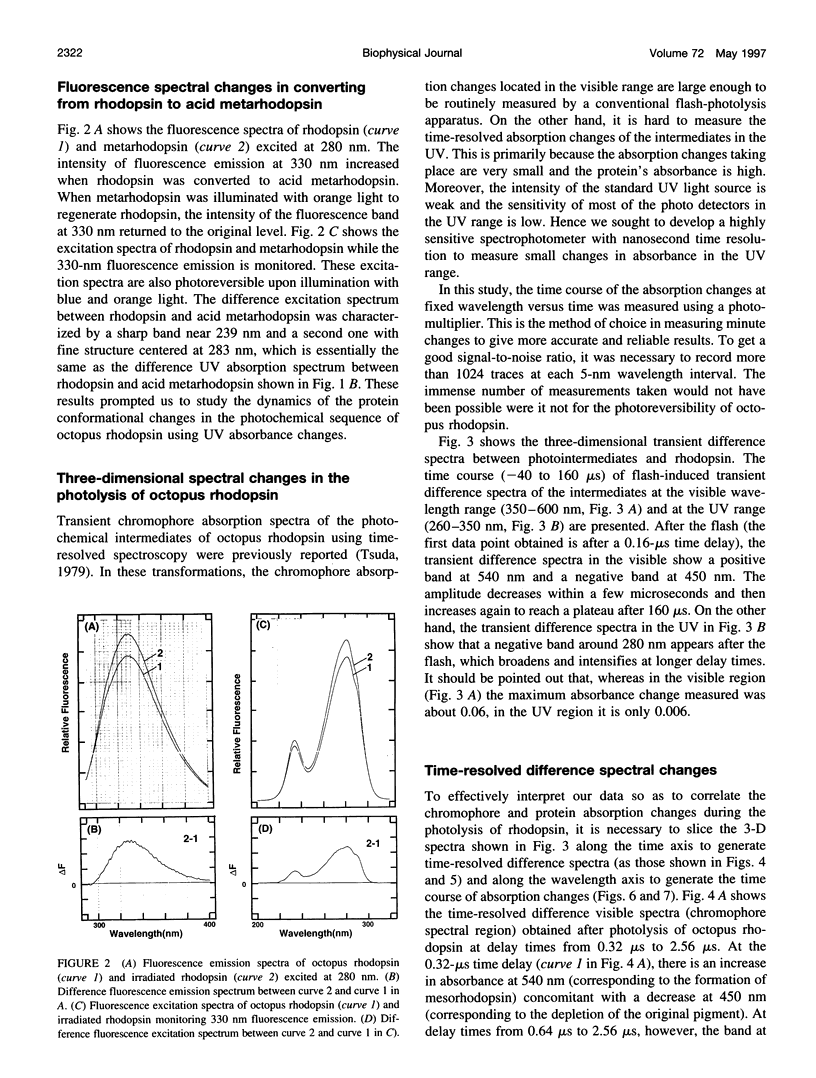
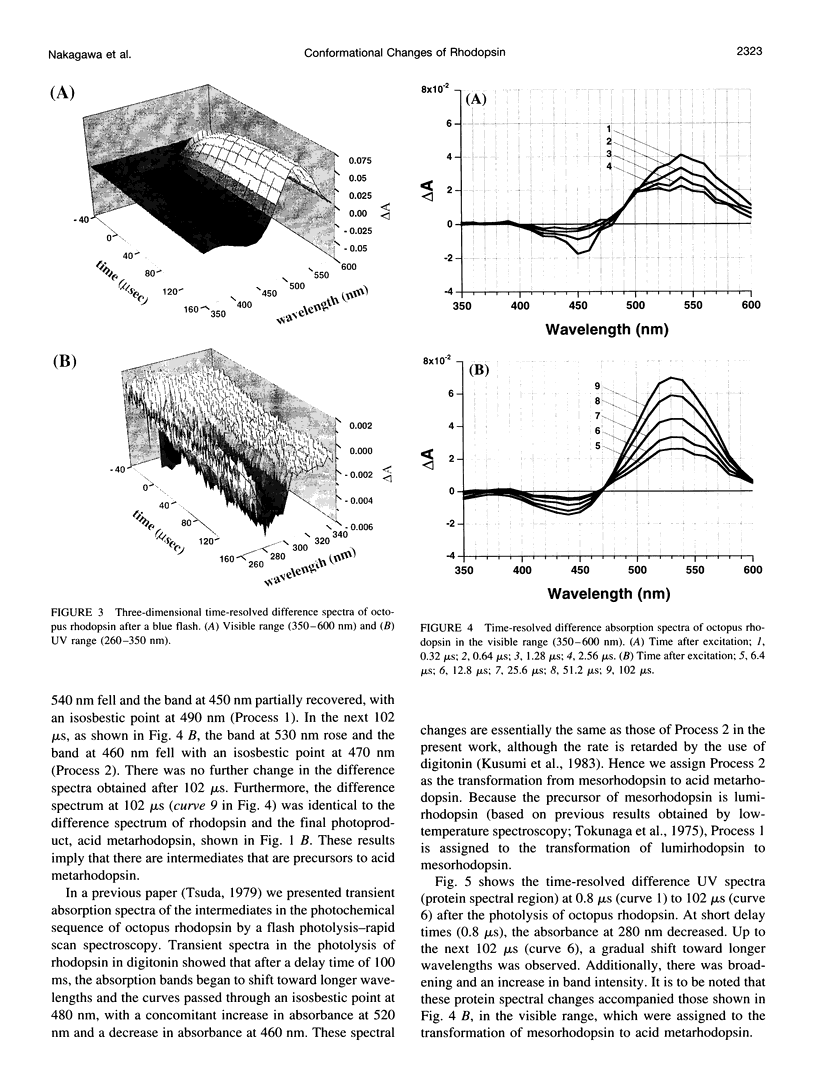
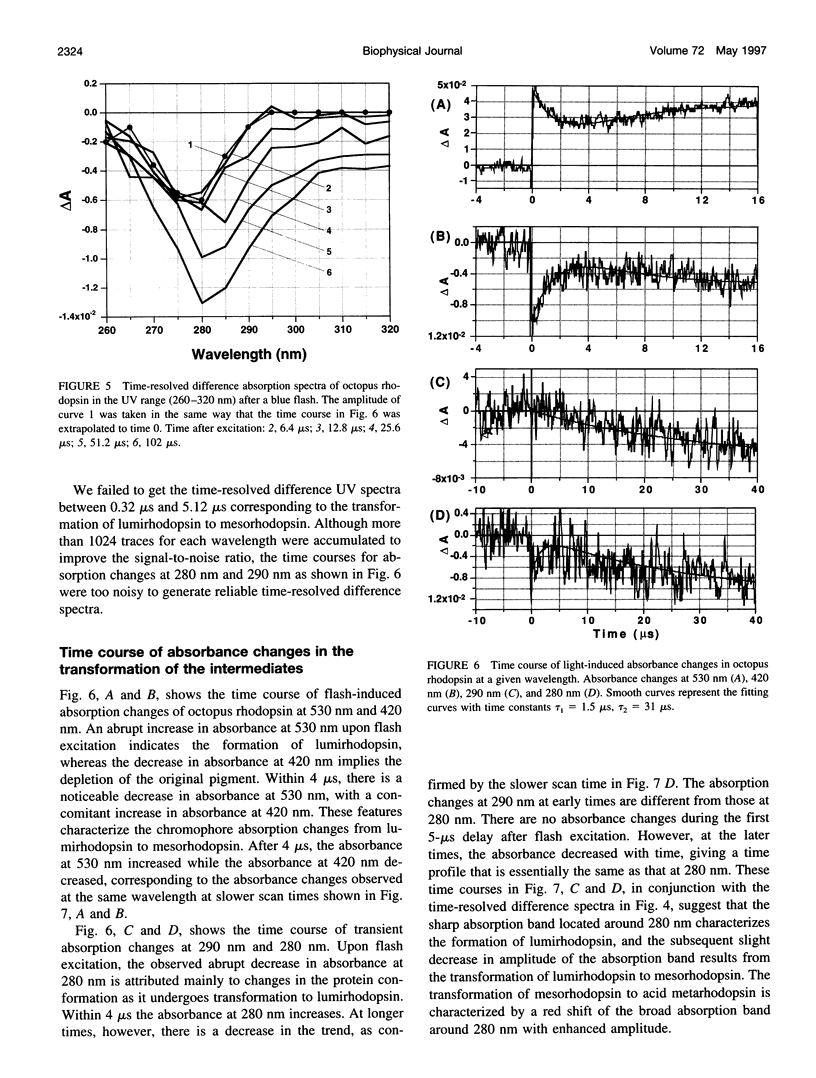
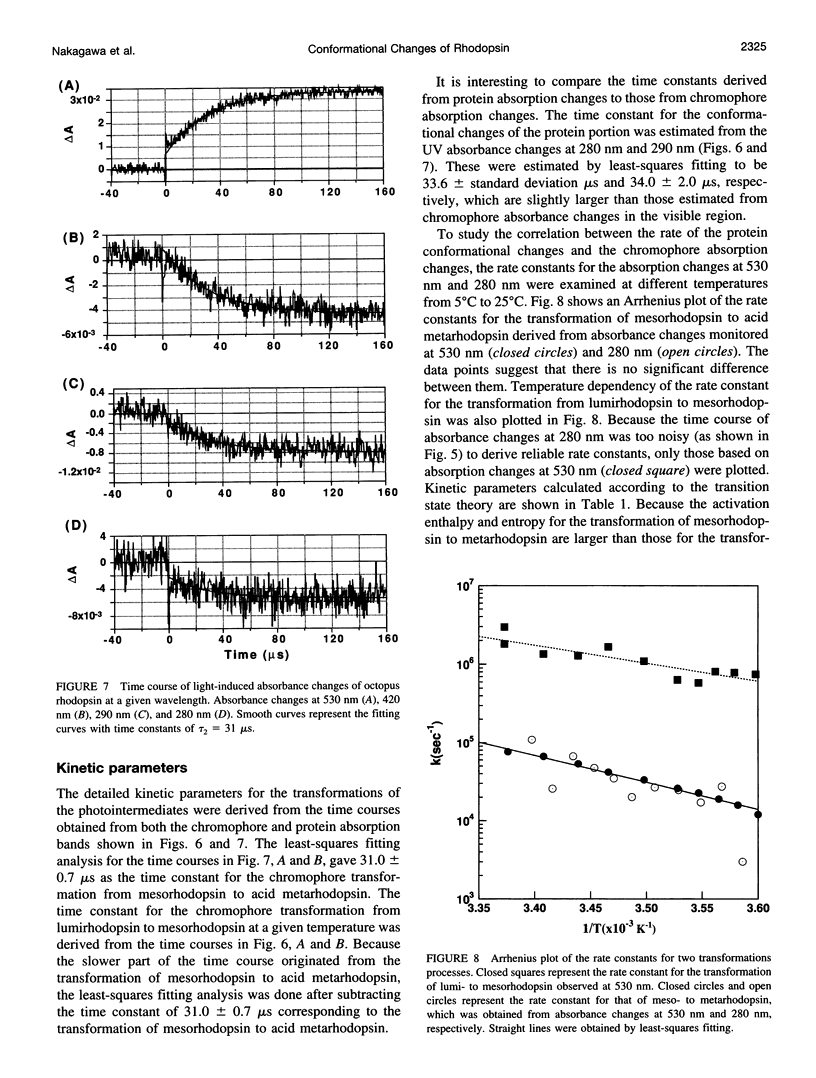
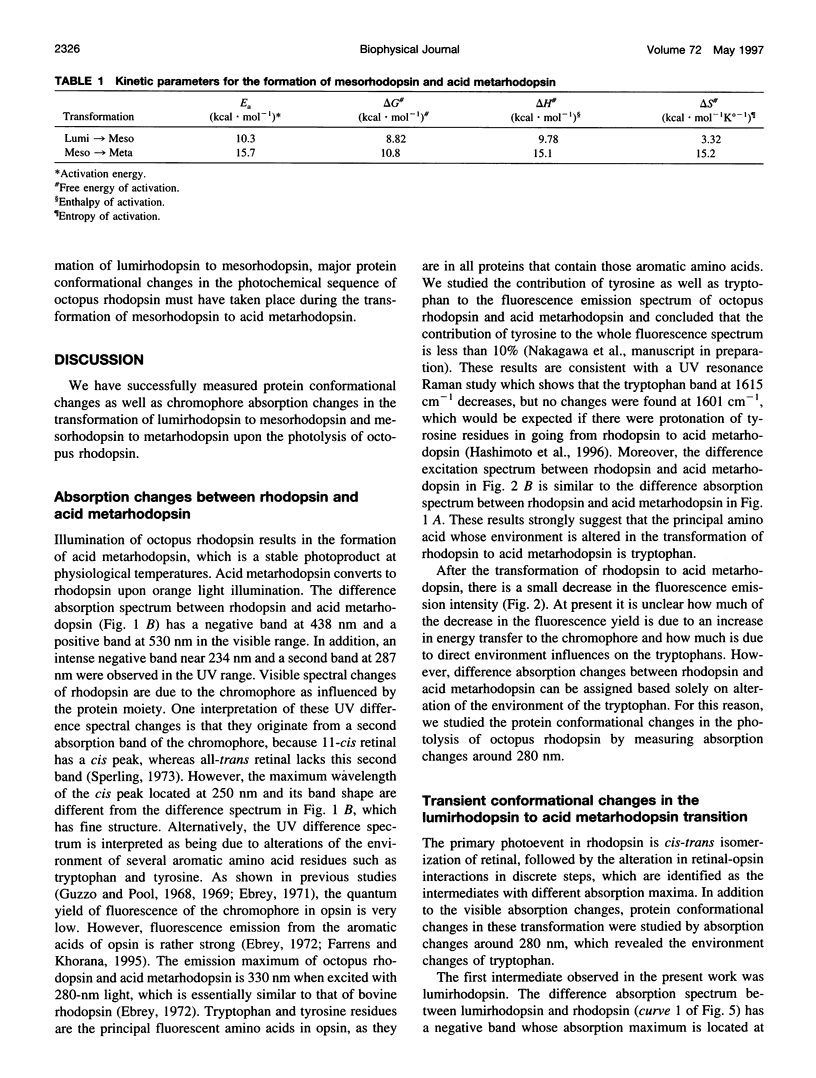
Light-induced protein conformational changes in the photolysis of octopus rhodopsin were measured with a highly sensitive time-resolved transient UV absorption spectrophotometer with nanosecond time resolution. A negative band around 280 nm in the lumirhodopsin minus rhodopsin spectra suggests that alteration of the environment of some of the tryptophan residues has taken place before the formation of lumirhodopsin. A small recovery of the absorbance at 280 nm was observed in the transformation of lumirhodopsin to mesorhodopsin. Kinetic parameters suggest that major conformational changes have taken place in the transformation of mesorhodopsin to acid metarhodopsin. In this transformation, drastic changes of amplitude and a shift of a difference absorption band around 280 nm take place, which suggest that some of the tryptophan residues of rhodopsin become exposed to a hydrophilic environment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K. A., Eisenstein L., Ebrey T. G., Tsuda M. A comparative study of the infrared difference spectra for octopus and bovine rhodopsins and their bathorhodopsin photointermediates. Biochemistry. 1989 Apr 18;28(8):3366–3373. doi: 10.1021/bi00434a036. [DOI] [PubMed] [Google Scholar]
- Cooper A., Dixon S. F., Tsuda M. Photoenergetics of octopus rhodopsin. Convergent evolution of biological photon counters? Eur Biophys J. 1986;13(4):195–201. doi: 10.1007/BF00260367. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. Energy transfer in rhodopsin, N-retinyl-opsin, and rod outer segments. Proc Natl Acad Sci U S A. 1971 Apr;68(4):713–716. doi: 10.1073/pnas.68.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G. The fluorescence from the tryptophans of rhodopsin. Photochem Photobiol. 1972 Jun;15(6):585–588. doi: 10.1111/j.1751-1097.1972.tb06269.x. [DOI] [PubMed] [Google Scholar]
- Farrens D. L., Khorana H. G. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem. 1995 Mar 10;270(10):5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]
- Guzzo A. V., Pool G. L. Fluorescence spectra of the intermediates of rhodopsin bleaching. Photochem Photobiol. 1969 Jun;9(6):565–570. doi: 10.1111/j.1751-1097.1969.tb07326.x. [DOI] [PubMed] [Google Scholar]
- Guzzo A. V., Pool G. L. Visual pigment fluorescence. Science. 1968 Jan 19;159(3812):312–314. doi: 10.1126/science.159.3812.312. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Takeuchi H., Nakagawa M., Tsuda M. Ultraviolet resonance Raman evidence for the absence of tyrosinate in octopus rhodopsin and the participation of Trp residues in the transition to acid metarhodopsin. FEBS Lett. 1996 Dec 2;398(2-3):239–242. doi: 10.1016/s0014-5793(96)01250-1. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Tsuda M. Resonance Raman spectra of octopus acid and alkaline metarhodopsins. Biochim Biophys Acta. 1980 Jul 24;624(1):211–217. doi: 10.1016/0005-2795(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Ohnishi S., Tsuda M. Light-induced conformational change of octopus rhodopsin as detected by a spin label method. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1635–1641. doi: 10.1016/s0006-291x(80)80086-6. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Tsuda M., Akino T., Ohnishi S., Terayama Y. Protein-phospholipid-cholesterol interaction in the photolysis of invertebrate rhodopsin. Biochemistry. 1983 Mar 1;22(5):1165–1170. doi: 10.1021/bi00274a027. [DOI] [PubMed] [Google Scholar]
- Liang J., Steinberg G., Livnah N., Sheves M., Ebrey T. G., Tsuda M. The pKa of the protonated Schiff bases of gecko cone and octopus visual pigments. Biophys J. 1994 Aug;67(2):848–854. doi: 10.1016/S0006-3495(94)80544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. W., Sakmar T. P. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state. Biochemistry. 1996 Aug 27;35(34):11149–11159. doi: 10.1021/bi960858u. [DOI] [PubMed] [Google Scholar]
- Masuda S., Morita E. H., Tasumi M., Iwasa T., Tsuda M. Infrared studies of octopus rhodopsin. Existence of a long-lived intermediate and the states of the carboxylic group of Asp-81 in rhodopsin and its photoproducts. FEBS Lett. 1993 Feb 15;317(3):223–227. doi: 10.1016/0014-5793(93)81280-d. [DOI] [PubMed] [Google Scholar]
- Ohtani H., Kobayashi T., Tsuda M., Ebrey T. G. Primary processes in photolysis of octopus rhodopsin. Biophys J. 1988 Jan;53(1):17–24. doi: 10.1016/S0006-3495(88)83061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Zolotarev A. S., Artamonov I. D., Bespalov I. A., Dergachev A. E., Tsuda M. Octopus rhodopsin. Amino acid sequence deduced from cDNA. FEBS Lett. 1988 May 9;232(1):69–72. doi: 10.1016/0014-5793(88)80388-0. [DOI] [PubMed] [Google Scholar]
- Pande A. J., Callender R. H., Ebrey T. G., Tsuda M. Resonance Raman study of the primary photochemistry of visual pigments. Hypsorhodopsin. Biophys J. 1984 Mar;45(3):573–576. doi: 10.1016/S0006-3495(84)84194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C., Pande A., Yue K. T., Callender R., Ebrey T. G., Tsuda M. Resonance Raman spectroscopy of octopus rhodopsin and its photoproducts. Biochemistry. 1987 Aug 11;26(16):4941–4947. doi: 10.1021/bi00390a009. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Shichida Y., Yoshizawa T. A new intermediate between lumirhodopsin and metarhodopsin in squid. FEBS Lett. 1975 Jul 15;55(1):229–232. doi: 10.1016/0014-5793(75)80998-7. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Tokunaga F., Ebrey T. G., Yue K. T., Marque J., Eisenstein L. Behaviour of octopus rhodopsin and its photoproducts at very low temperatures. Nature. 1980 Oct 2;287(5781):461–462. doi: 10.1038/287461a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M. Transient spectra of intermediates in the photolytic sequence of octopus rhodopsin. Biochim Biophys Acta. 1979 Mar 15;545(3):537–546. doi: 10.1016/0005-2728(79)90162-2. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Tsuda T., Hirata H. Cyclic nucleotides and GTP analogues stimulate light-induced phosphorylation of octopus rhodopsin. FEBS Lett. 1989 Oct 23;257(1):38–40. doi: 10.1016/0014-5793(89)81780-6. [DOI] [PubMed] [Google Scholar]



