Abstract
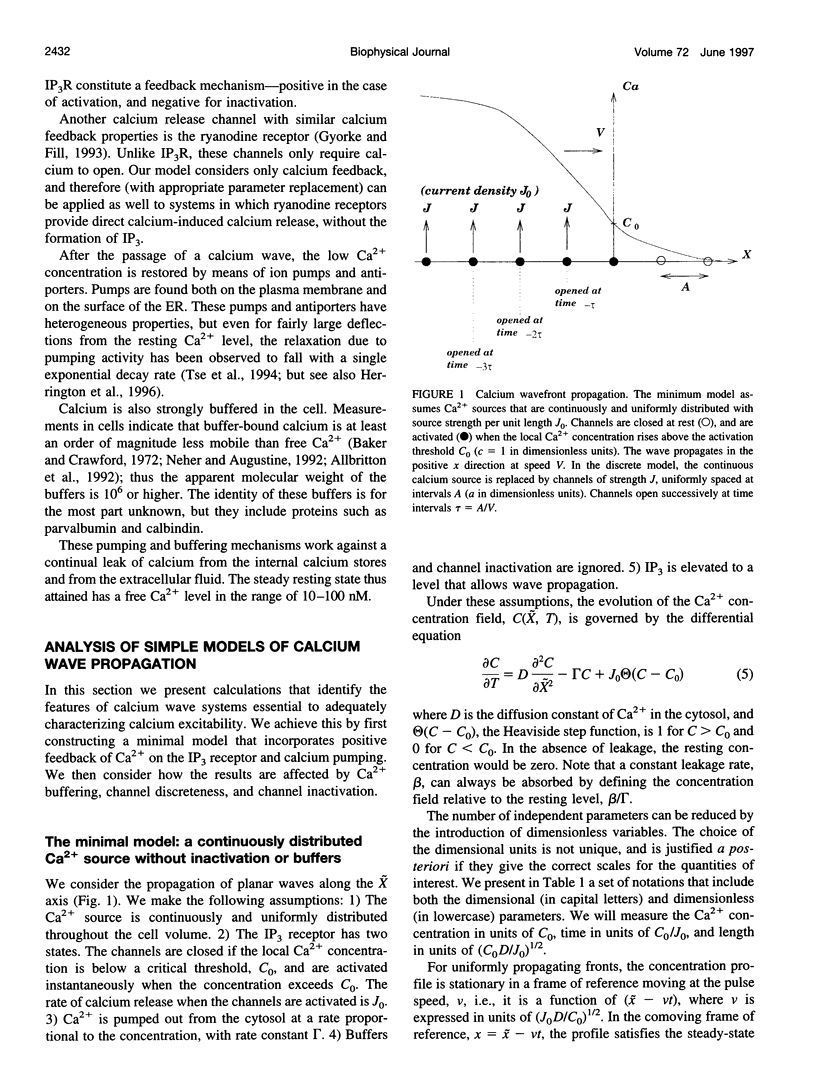
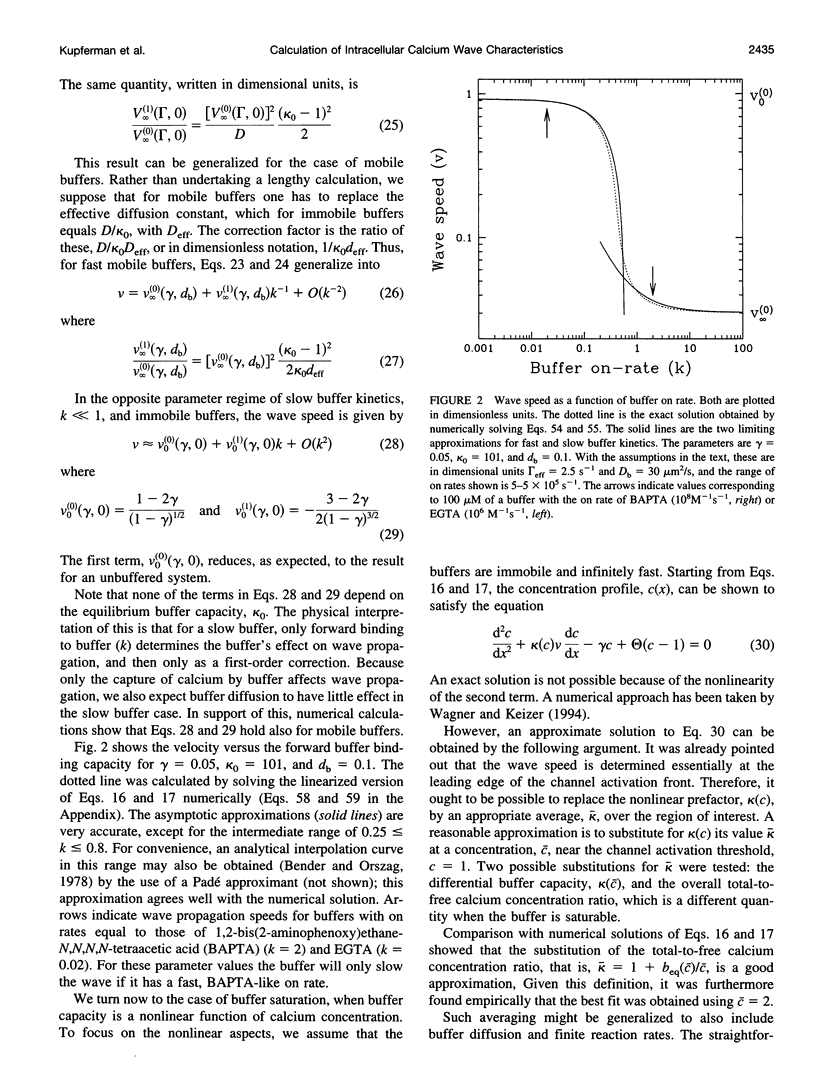
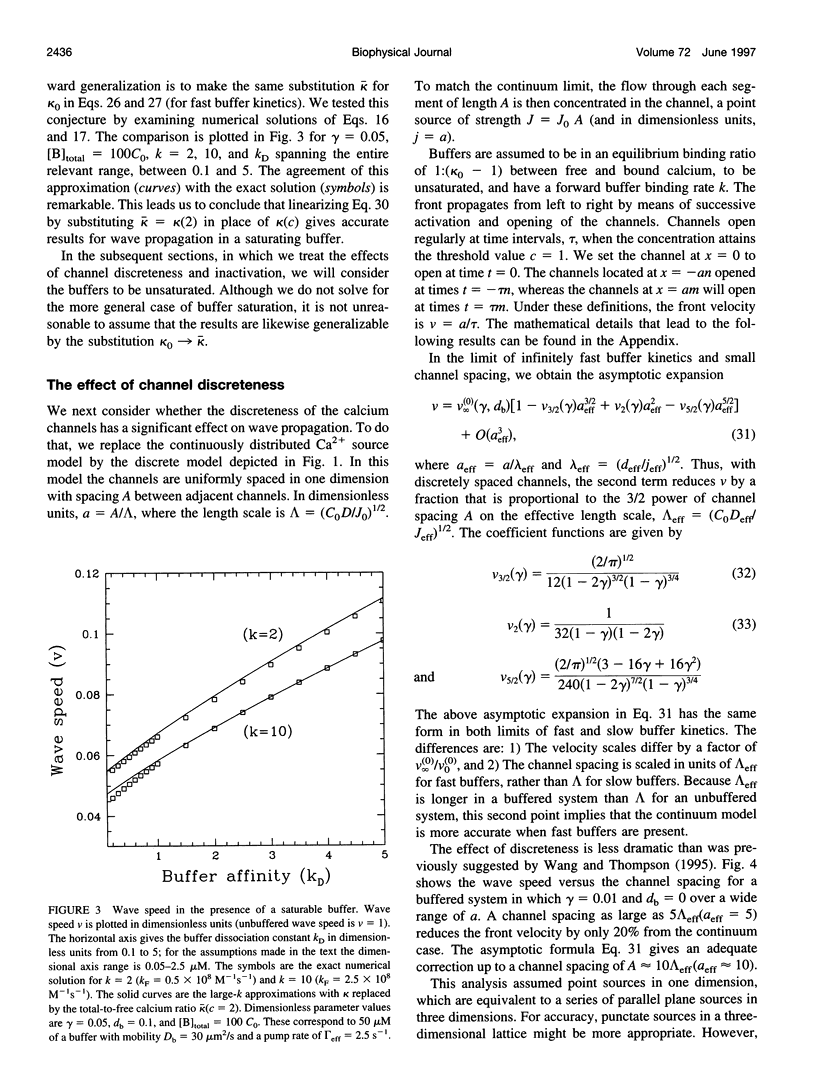
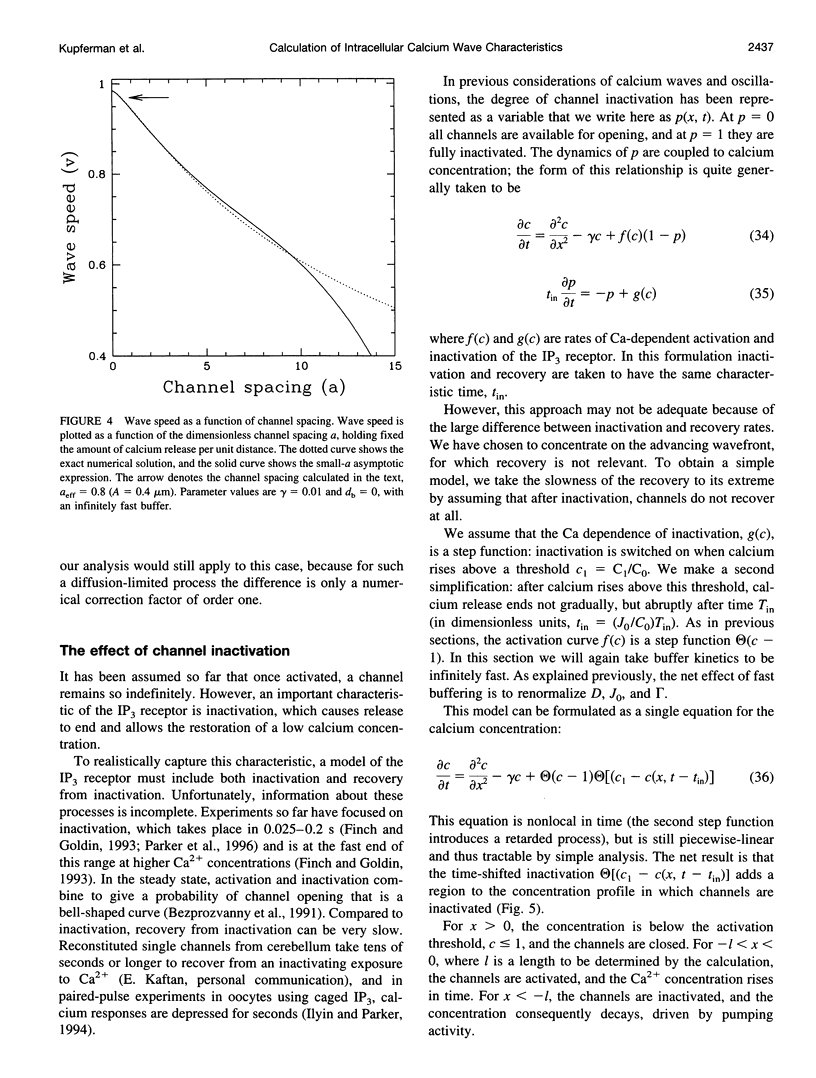
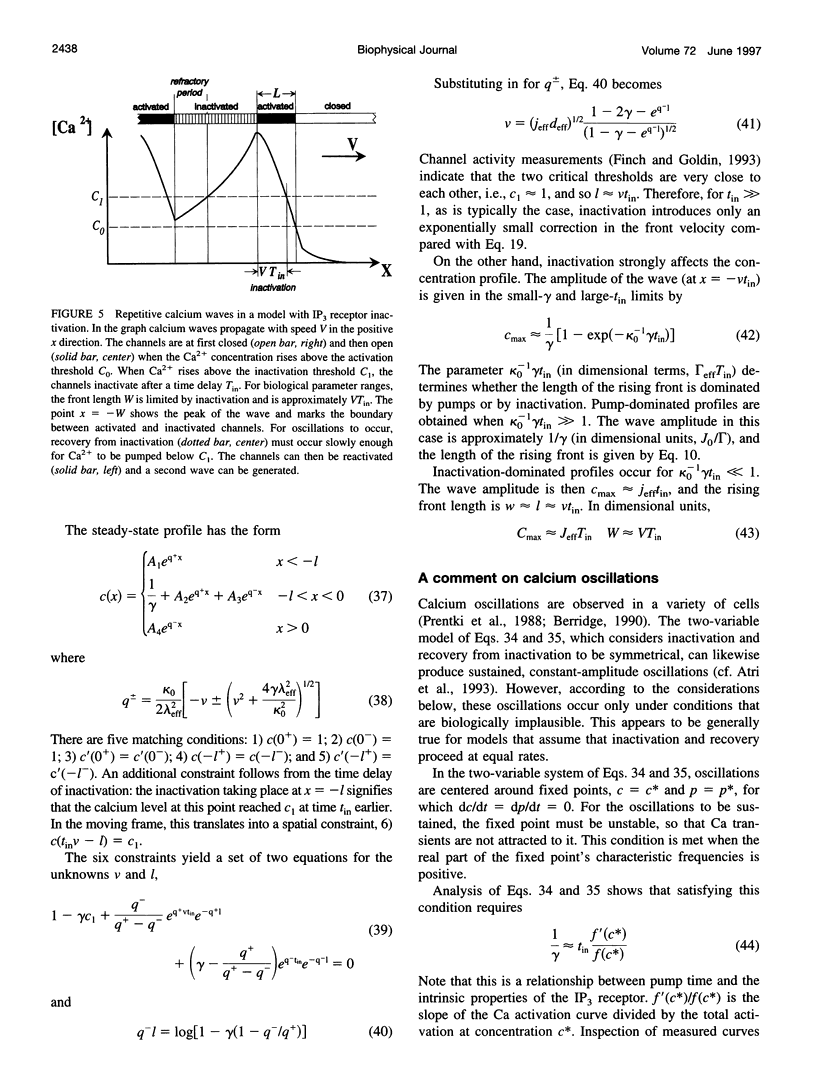
We present a theoretical analysis of intracellular calcium waves propagated by calcium feedback at the inositol 1,4,5-trisphosphate (IP3) receptor. The model includes essential features of calcium excitability, but is still analytically tractable. Formulas are derived for the wave speed, amplitude, and width. The calculations take into account cytoplasmic Ca buffering, the punctate nature of the Ca release channels, channel inactivation, and Ca pumping. For relatively fast buffers, the wave speed is well approximated by V(infinity) = (J(eff)D(eff)/C0)1/2, where J(eff) is an effective, buffered source strength; D(eff) is the effective, buffered diffusion constant of Ca; and C(0) is the Ca threshold for channel activation. It is found that the saturability and finite on-rate of buffers must be taken into account to accurately derive the wave speed and front width. The time scale governing Ca wave propagation is T(r), the time for Ca release to reach threshold to activate further release. Because IP3 receptor inactivation is slow on this time scale, channel inactivation does not affect the wave speed. However, inactivation competes with Ca removal to limit wave height and front length, and for biological parameter ranges, it is inactivation that determines these parameters. Channel discreteness introduces only small corrections to wave speed relative to a model in which Ca is released uniformly from the surface of the stores. These calculations successfully predict experimental results from basic channel and cell parameters and explain the slowing of waves by exogenous buffers.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca(2+)-induced Ca2+ release. Biophys J. 1994 Dec;67(6):2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Herrington J., Park Y. B., Babcock D. F., Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996 Jan;16(1):219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Ilyin V., Parker I. Role of cytosolic Ca2+ in inhibition of InsP3-evoked Ca2+ release in Xenopus oocytes. J Physiol. 1994 Jun 15;477(Pt 3):503–509. doi: 10.1113/jphysiol.1994.sp020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9883–9887. doi: 10.1073/pnas.88.21.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M. S., Keizer J. On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys J. 1995 Nov;69(5):2139–2153. doi: 10.1016/S0006-3495(95)80088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y., Ilyin V. Fast kinetics of calcium liberation induced in Xenopus oocytes by photoreleased inositol trisphosphate. Biophys J. 1996 Jan;70(1):222–237. doi: 10.1016/S0006-3495(96)79565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Glennon M. C., Thomas A. P., Morris R. L., Matschinsky F. M., Corkey B. E. Cell-specific patterns of oscillating free Ca2+ in carbamylcholine-stimulated insulinoma cells. J Biol Chem. 1988 Aug 15;263(23):11044–11047. [PubMed] [Google Scholar]
- Rinzel J., Keller J. B. Traveling wave solutions of a nerve conduction equation. Biophys J. 1973 Dec;13(12):1313–1337. doi: 10.1016/S0006-3495(73)86065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Girard S., Clapham D. Calcium wave propagation by calcium-induced calcium release: an unusual excitable system. Bull Math Biol. 1993 Mar;55(2):315–344. doi: 10.1007/BF02460886. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Kalachev L. V. A profile analysis of propagating calcium waves. Cell Calcium. 1994 Apr;15(4):289–296. doi: 10.1016/0143-4160(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Keizer J., Sanderson M. J. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J. 1995 Nov;9(14):1463–1472. doi: 10.1096/fasebj.9.14.7589988. [DOI] [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys J. 1994 Dec;67(6):2223–2235. doi: 10.1016/S0006-3495(94)80707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Alousi A. A., Thompson S. H. The lifetime of inositol 1,4,5-trisphosphate in single cells. J Gen Physiol. 1995 Jan;105(1):149–171. doi: 10.1085/jgp.105.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Thompson S. H. Local positive feedback by calcium in the propagation of intracellular calcium waves. Biophys J. 1995 Nov;69(5):1683–1697. doi: 10.1016/S0006-3495(95)80086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



