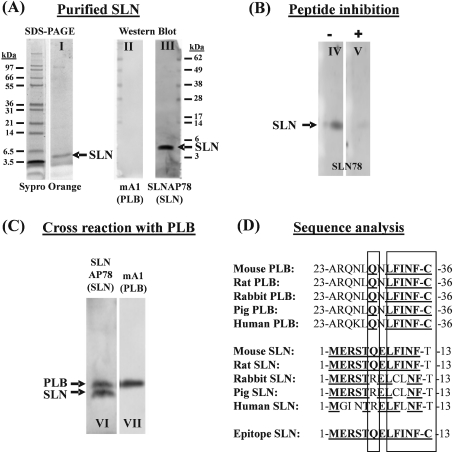
Figure 3. Characterization of the anti-SLN antibody.
(A) Purified SLN (0.1 μg) was visualized with Sypro Orange after SDS/PAGE (lane I). After transfer on to a PVDF membrane, the SLNAP78 antibody recognizes the purified SLN (lane III). No PLB is present in the purified SLN (lane II) as shown by incubation of the blot with an anti-PLB antibody. (B) Addition of antigenic peptide in solution (+) during primary antibody incubation (lane V) can reduce the antibody reaction with purified SLN (−) (lane IV). (C) The SLNAP78 antibody cross-reacts with PLB. Homogenate of mouse atria (70 μg) was transferred to a blot and stained in parallel with the SLNAP78 (lane VI) and anti-PLB antibody (lane VII). PLB (apparent molecular mass 5 kDa) and SLN (apparent molecular mass 4 kDa) were discriminated by size. (D) Comparison of the amino acid sequence of homologous parts of PLB, SLN and the epitope used to develop anti-SLN antibodies. Bold and underlined amino acids are identical with the epitope. As indicated by the two rectangles, the C-terminal part of the PLB sequence shows striking similarity to the corresponding mouse and rat SLN sequence, and to the epitope.