Abstract
ProTGFα (transforming growth factor α precursor) maturation and conversion into soluble TGFα is a complex process that involves three proteolytic steps. One, that occurs co-translationally, eliminates the signal sequence. Another, occurring at the juxtamembrane domain, solubilizes TGFα. A third cleavage removes the N-terminal extension of proTGFα. This latter step has been poorly studied, mainly because of the rapid kinetics of this cleavage. In the present study, we have designed a strategy to analyse several aspects regarding this N-terminal cleavage. In vivo treatment with the hydroxamate-based metalloprotease inhibitors BB3103 or TAPI-2 (tumour necrosis factor-α protease inhibitor 2) reversibly induced accumulation of forms of proTGFα that included the N-terminal extension. N-terminal shedding was rapid, and occurred at the cell surface. However, the machinery responsible for the N-terminal cleavage was inactive in other cellular sites, such as the endoplasmic reticulum. Experiments of proTGFα expression and maturation in cells deficient in TACE (tumour-necrosis-factor-α-converting enzyme) activity indicated that this protease was dispensable for N-terminal processing of proTGFα in vivo, but was required for regulated cleavage at the C-terminus. These findings indicate that TACE is not involved in N-terminal processing of proTGFα, and suggest differences in the machineries that control the cleavage at both ends of TGFα within its precursor.
Keywords: growth factor, metalloprotease, N-terminal shedding, secretase, transforming growth factor α (TGF-α)
Abbreviations: ADAM, a disintegrin and metalloprotease; CHO, Chinese-hamster ovary; CHX, cycloheximide; DMEM, Dulbecco's modified Eagle's medium; E64, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide; EGF, epidermal growth factor; ER, endoplasmic reticulum; FBS, foetal bovine serum; GST, glutathione S-transferase; HA, haemagglutinin; HBSS, Hanks balanced salt solution; HEK, human embryonic kidney; PKC, protein kinase C; TACE, tumour-necrosis-factor-α-converting enzyme; TAPI-2, tumour necrosis factor-α protease inhibitor 2; TBST, Tris-buffered saline with Tween 20; (pro)TGFα, transforming growth factor α (precursor)
INTRODUCTION
TGFα (transforming growth factor α) is a soluble protein that was initially isolated from the culture medium of transformed cells by its ability to stimulate anchorage-independent cell growth [1]. Later, multiple studies indicated that increased function or expression of TGFα was associated with the development/progression of various types of cancer. Thus TGFα has been detected in several tumours [2,3], and transgenic overexpression of this factor in mice results in hyperplasia of the mammary gland, and pancreatic and liver carcinomas [4–6].
TGFα is biosynthesized as a larger transmembrane protein termed proTGFα (TGFα precursor) [7,8]. This precursor protein includes several different domains (see Figure 1A) that play distinct roles in the biology of proTGFα [9]. The ectodomain of this precursor molecule includes an N-terminal signal sequence that allows translocation of the nascent polypeptide chain through the membrane of the ER (endoplasmic reticulum) [7,8], an N-terminal extension [10], the TGFα region that includes the EGF (epidermal growth factor)-like module [11] and a linker sequence that connects the ectodomain to the transmembrane domain. The intracellular domain is short and has been implicated in proper sorting and cleavage of proTGFα [12,13].
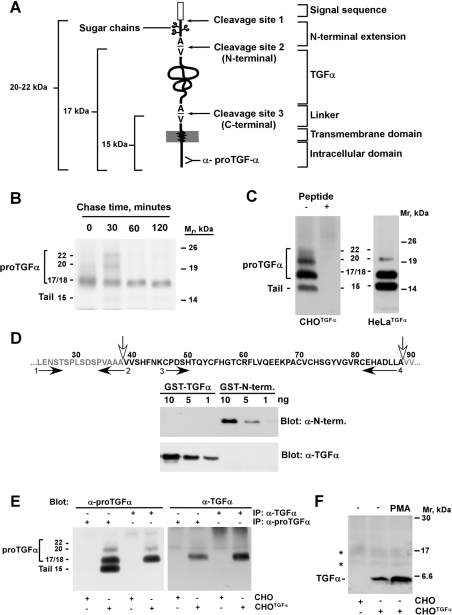
Figure 1. Molecular forms of proTGFα expressed by CHO and HeLa cells.
(A) Schematic representation of the different domains of proTGFα, and the three cleavage sites. The site recognized by the anti-proTGFα antibody at the C-terminus of the precursor protein is shown. (B) Pulse–chase experiment of proTGFα. CHOTGFα cells were pulsed for 20 min with [35S]cysteine, and then chased for the indicated times. Cell lysates were immunoprecipitated with the anti-proTGFα antibody, and the precipitates were analysed by SDS/PAGE followed by autoradiography. The position of the molecular-mass markers is indicated at the right, and the molecular mass of the different proTGFα forms are indicated at the left. (C) Forms of proTGFα in CHOTGFα and HeLaTGFα cells. Lysates were prepared, and precipitated with the anti-proTGFα antibody, followed by Western blotting with the same antibody. Where indicated, the precipitates were incubated also with 1 μg of the peptide against which the anti-proTGFα was raised. Molecular masses are in kDa. (D) Generation of anti-proTGFα ectodomain antibodies. The sequence at the top includes the N-terminal extension of proTGFα (grey letters, at the left) and the TGFα module (black letters). The vertical white filled arrows indicate the sites of N-terminal cleavage, between residues 38 and 39; and the C-terminal cleavage, between residues 88 and 89 of proTGFα respectively. The horizontal arrows indicate the position of the primers used to raise DNA fragments from rat proTGFα to generate the GST–ectodomain fusion protein (primers 1 and 4) that was injected into rabbits to generate antibodies. The antibodies specific to the N-terminus (N-term.) were purified by constructing a GST–N-terminus fusion protein using primers 1 and 2; and the antibodies against TGFα were purified from the same serum using a GST–TGFα fusion protein generated with primers 3 and 4. The resulting antibodies were tested for their specificity by Western blotting using different amounts of GST–TGFα or GST–N-terminus protein, as indicated in the gels shown. (E) Extracts from CHO or CHOTGFα cells were immunoprecipitated (IP) with anti-proTGFα or anti-TGFα antibodies, and then Western blots were probed with anti-proTGFα (left-hand panel), or anti-TGFα antibodies (right-hand panel). Molecular masses are in kDa. (F) Release of soluble TGFα into the culture medium. Medium from CHO or CHOTGFα cells (conditioned for 24 h) was concentrated and then soluble TGFα was detected in Western blots with the anti-TGFα antibody. Where indicated, PMA (1 μM) was added to the cells for 30 min before harvesting of the culture medium. The asterisks indicate two non-specific bands. Molecular masses are in kDa.
Three proteolytic events contribute to the full maturation of proTGFα. The first (cleavage site 1 in Figure 1A) involves co-translational removal of the signal peptide by signal peptidases [14,15]. This is expected to be the faster proteolytic processing of proTGFα and occurs upon entry of the precursor peptide to the secretory route in the ER. This cleavage generates a form of proTGFα that contains an N-terminal extension. The two other processing steps occur between alanine and valine peptide bonds, and result in either the elimination of the N-terminal proTGFα extension (processing at cleavage site 2 in Figure 1A), or cleavage at the C-terminus of TGFα within its precursor (processing at cleavage site 3 in Figure 1A).
Much attention has been paid to the shedding that occurs in the linker region at cleavage site 3, and which is responsible for the solubilization of TGFα [9,16]. Processing at this site is slow under resting conditions, but may be accelerated by treatments that affect several intracellular signalling pathways [17–19]. Analyses of the cellular location where processing at the C-terminus occurs indicated that the machinery responsible for this shedding event is only functional at the plasma membrane, but requires the presence of cytosolic components [12,20]. Studies aimed at the identification of the proteases responsible for the cleavage of proTGFα at the C-terminus have shown that metalloproteases have a predominant role in the control of the shedding of TGFα [21]. Studies in this direction have indicated that ADAM17 (a disintegrin and metalloprotease 17)/TACE (tumour-necrosis-factor-α-converting enzyme) may act as the major metalloprotease in the shedding of TGFα [22–24]. Thus, in animals deficient in TACE activity, release of TGFα is severely reduced [23]. Furthermore, these animals present a phenotype that mimics, in some aspects, the TGFα-knockout mice [23]. In addition, in vitro measurements of TGFα release have indicated that efficient shedding of TGFα requires the activity of TACE [22]. However, a number of observations indicate that other proteases, in addition to TACE, may control the shedding of TGFα. In fact, even though the release of TGFα is severely decreased in animals with impaired TACE activity, a residual amount of TGFα is found as a soluble form in the culture medium of fibroblasts derived from these animals [23]. Also, in cells derived from animals deficient in TACE activity, shedding of TGFα can be increased by certain treatments, such as APMA (4-aminophenylmercuric acetate), indicating that proteases other than TACE may act in the regulation of the cleavage of proTGFα [25].
Another cleavage event, occurring at cleavage site 2, removes the N-terminal extension of proTGFα. This region is glycosylated, and links the signal sequence to the N-terminus of TGFα [10,26]. Because of the heterogeneous glycosylation that occurs at this region, several molecular forms of soluble TGFα have been recovered from the culture medium of cells expressing this factor, and being cleaved at site 3 more efficiently than at site 2 [27,28]. The characteristics of the proteolytic machinery that are responsible for the shedding of the N-terminal extension are poorly known. One of the questions that remains to be elucidated is the cellular location at which N-terminal cleavage occurs. Pulse–chase experiments performed in cells that express proTGFα have indicated that the N-terminal processing activity rapidly removes the N-terminus of proTGFα [10,17]. This rapid disappearance, together with the fact that forms of proTGFα with the N-terminal extension are difficult to detect, has raised the possibility that the N-terminal shedding may occur shortly after proTGFα biosynthesis, or during transit of the precursor to the plasma membrane [10,17]. In this respect, a potential cellular site that could participate in N-terminal proTGFα shedding is the ER. This is supported not only by the rapid disappearance of the N-terminus of proTGFα, but also because this compartment contains the proteolytic machinery that processes proTGFα at site 1, removing its signal sequence. In addition, it is expected that, since N-terminal cleavage occurs in a luminal space, the N-terminal secretase and proTGFα should coincide in the ER, at least during their synthesis. However, indirect data indicate that N-terminal shedding may occur outside this cellular compartment. Thus forms of proTGFα containing the N-terminal extension have been found to be sensitive to exogenous elastase, indicating that these forms may reach the plasma membrane [10]. In addition, since cleavage at the N-terminus and at the C-terminus occurs between Ala–Val peptide bonds, and cleavage at the C-terminus occurs at the plasma membrane [12], the possibility that the same cell-surface protease may act at both sites has been proposed [22]. In this direction, recent in vitro experiments have indicated that TACE may be the major N-terminal processing enzyme [22], and that other secretases, such as ADAM10 may also cleave at this site [29]. However, TACE is mainly located in intracellular compartments, and only a minor proportion reaches the plasma membrane [30].
In the present study, we have developed a strategy to overcome the problem of the rapid removal of the N-terminal extension. This has allowed us to study the cellular site and the potential importance of TACE as an N-terminal secretase. We show that the proteolytic activity that processed proTGFα at the N-terminus may act at the cell surface, but not in intracellular compartments, such as the ER. In addition, we report that the N-terminal secretase activity is sensitive to certain metalloprotease inhibitors, and that this inhibition is reversible. Finally, we show that N-terminal shedding of proTGFα occurs in the absence of TACE. However, efficient resting and regulated cleavage at the C-terminus of TGFα requires the presence of active forms of this protease.
EXPERIMENTAL
Reagents and immunochemicals
PMA, PMSF, tosylphenylalanylchloromethane, proteinase K, 1,10-phenanthroline, EDTA, soybean trypsin inhibitor, E64 [N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide], ALLN (N-acetyl-L-leucyl-L-leucyl-L-norleucinal), CHX (cycloheximide), brefeldin A, aprotinin, pepstatin, leupeptin and polybrene were from Sigma. BB3103 and TAPI-2 (tumour necrosis factor-α protease inhibitor 2) were generously provided by British Biotech and Amgen-Immunex respectively. G418 was from Invitrogen. Glutathione–Sepharose was from Amersham Biosciences.
The proTGFα-specific antiserum was raised in rabbits against a synthetic peptide that included amino acids 71–88 [17]. The anti-TACE antibody was generated in rabbits against the 14 C-terminal amino acids of the cytosolic tail of human TACE [31]. Antibodies against proTGFα or TACE were purified by affinity chromatography using peptide–Sepharose columns as described in [31].
We also generated two different antibodies against the ectodomain of proTGFα, that recognized the N-terminus of proTGFα, or the EGF-like module that corresponds to TGFα. For this, rabbits were immunized with a GST (glutathione S-transferase) fusion protein that included amino acids 23–88 of the ectodomain of proTGFα (Figure 1D). The antibodies against the N-terminus, or the TGFα domain, were purified from the antiserum by affinity chromatography. A first pre-purification step using GST coupled to Sepharose CL-4B was carried out to remove the anti-GST antibodies. Three purification rounds were necessary to eliminate any anti-GST antibodies. The flow-through was then passed over a column that contained the GST–N-terminus protein (amino acids 23–38 of proTGFα) coupled to Sepharose CL-4B, in order to purify the corresponding anti-N-terminus antibodies. Then, the antiserum was passed through a third column that contained the fusion protein GST–TGFα (amino acids 46–88) coupled to Sepharose CL-4B, to purify the anti-TGFα antibodies.
The generation in bacteria of the different GST-fusion proteins was performed according to standard procedures [32]. The oligonucleotides (as numbered in Figure 1D) used to generate the distinct fusion proteins were: 1, 5′-AATGGATCCCTGGAGAACAGCACG-3′; 2, 5′-CAGAAGCTTTGCAGCCGCCACGGG-3′; 3, 5′-AATGGATCCTGCCCAGATTCCCAC-3′, and 4, 5′-CAGAAGCTTCAGGAGATCTGCATG-3′. The different oligonucleotides were used to generate different DNA fragments from rat proTGFα by PCR, and these fragments were digested with BamHI and HindIII, and subcloned into the pKG vector for the production of recombinant fusion proteins.
Cell culture and transfections
All cell lines were cultured at 37 °C in a humidified air atmosphere (5% CO2/95% air). Cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 100 units/ml penicillin G and 100 μg/ml streptomycin, and 5% (v/v) FBS (foetal bovine serum) [CHO (Chinese-hamster ovary), M2, TACEΔZn/ΔZn and HeLa cells] or 10% (v/v) FBS [HEK-293T (human embryonic kidney) cells]. CHO cells expressing proTGFα (CHOTGFα cells) have been described in [33]. CHO cells expressing the proTGFα construct tagged with an HA (haemagglutinin) peptide at the N-terminus of the mature growth factor, and the corresponding shedding-defective mutants (M2 cells) have been described elsewhere [21,34]. For the expression of pro-TGFα in HeLa cells, the pVAO vector containing the rat proTGFα cDNA was co-transfected with pCDNA3 by calcium phosphate, and clones were selected with 500 μg/ml G418. The expression of proTGFα in individual clones was analysed by Western blotting with the anti-proTGFα affinity-purified antibodies. Expression of proTGFα in TACEΔZn/ΔZn fibroblasts was performed by co-transfection of pVAO-proTGFα with a puromycin resistance vector (pBabe-puro). After 3 weeks of selection in 2, 3 or 4 μg/ml puromycin, resistant clones were analysed for proTGFα expression by Western blotting with the anti-proTGFα antibody.
Retrovirus production and infection
For the generation of retroviruses, HEK-293T cells were plated in 60-mm-diameter dishes (1.8×106 cells in 3 ml of DMEM with 10% FBS) and were allowed to attach overnight. At 5 min before transfection, 25 μM chloroquine was added to each plate. The transfection solution contained DNA [2.5 μg of pMD-G, 5 μg of pNGVL-MLV-gag-pol, 3 μg of retroviral vector (pLZR-TACE-IRES-GFP or pLZR-IRES-GFP alone for the mock transfection)], 61 μl of 2 M CaCl2, and double-distilled water to make it up to 500 μl. After mixing, 0.5 ml of 2× HBSS (Hanks balanced salt solution), pH 7.0, was added, and the solution was bubbled for 15 s. The HBSS/DNA mixture was then dropped on to the cell monolayers. After 8 h, the medium was replaced with fresh complete culture medium that, 24 to 32 h post-transfection, was again replaced with 3 ml of fresh virus-collecting medium. At 24 h after the medium change, the supernatant from transfected cells was collected and centrifuged at 1000 g for 5 min. At 1 day before infection, TACEΔZn/ΔZn−TGFα cells were plated in 60-mm-diameter dishes and allowed to attach. Cells were infected with viral supernatants containing polybrene at 6 μg/ml. The following day, the medium was changed for TACEΔZn/ΔZn−TGFα cells that were infected overnight, and cleavage studies were performed between 3 and 5 days after the starting of the infection. The amount of infected TACE was analysed by Western blotting of cell lysates with the anti-TACE affinity-purified antibodies.
Immunoprecipitation and Western blotting
Cells were washed with PBS and lysed in ice-cold lysis buffer (140 mM NaCl, 10 mM EDTA, 10% glycerol, 1% Nonidet P40, 20 mM Tris/HCl, pH 7.5, 1 μM pepstatin, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF and 1 mM sodium orthovanadate). After scraping the cells from the dishes, samples were centrifuged at 10000 g for 10 min at 4 °C, and supernatants were transferred to new tubes with rabbit anti-proTGFα antibodies and Protein A–Sepharose. Immunoprecipitations were performed at 4 °C for at least 2 h, and the immunocomplexes were recovered by a 10 s centrifugation at 10000 g, followed by three washes with 1 ml of ice-cold lysis buffer. Samples were then boiled in electrophoresis sample buffer and loaded on 10–15% gradient SDS/PAGE gels. After transfer to Immobilon membranes, filters were blocked for at least 1 h in TBST [Tris-buffered saline (20 mM Tris/HCl, pH 7.5, and 150 mM NaCl) with 0.2% Tween 20] and with 1% BSA and then incubated for 1–12 h with the anti-proTGFα antibody. After washing with TBST, filters were incubated with horseradish-peroxidase-conjugated secondary antibodies for 30 min, and bands were visualized by a luminol-based detection system with p-iodophenol enhancement as described previously [35]. Unless otherwise indicated, the blots shown in the Figures are representative of an experiment which was repeated at least twice.
Detection of soluble TGFα
CHO or CHOTGFα cell monolayers were washed three times (20 min each) with serum-free culture medium and then incubated in the same medium with the corresponding treatments. Media (2 ml) was collected and concentrated through an Amicon Ultra 5000 Mr cut-off device (Millipore, Bedford, MA, U.S.A.) to a final volume of 100 μl. Samples were loaded on an SDS/10–15% PAGE gel, and the separated proteins in the gel were blotted on to PVDF membranes that were probed with the affinity-purified anti-TGFα antibodies. Visualization of TGFα in the blots was carried out by using the ECL® (enhanced chemiluminescence) Plus Western blotting detection system (Amersham Biosciences).
Metabolic labelling
For metabolic labelling, CHOTGFα cells were plated in 60-mm-diameter dishes and allowed to reach 70–90% confluence. When ready for the experiments, cells were shifted to pre-warmed cysteine- and serum-free MEM (minimum essential medium). After two 20-min washes in this medium, cells were labelled for 15–20 min with 200 μCi/ml of [35S]cysteine. The medium was then replaced with DMEM, and incubations were continued for the indicated times. Cells were then rinsed, recovered in lysis buffer by scraping, and centrifuged at 10000 g. Immunoprecipitations were carried overnight with rabbit anti-proTGFα antibodies as described above. Immunocomplexes were recovered with Protein A–Sepharose, washed and analysed on SDS/10–15% PAGE gels. Gels were fixed and fluorographed using Enlightening (DuPont/NEN).
To analyse the fate of proTGFα located at the cell surface, CHO cells expressing HA–proTGFα were labelled with 500 μCi/ml of [35S]cysteine for 20 min, and the label was chased in complete medium for 30 min. Cells were then washed with cold PBS and incubated at 4 °C with 10 μg/ml of anti-HA antibodies in PBS containing 5% BSA for 1 h. Unbound anti-HA antibodies were washed with PBS, and then cells were incubated for various periods of time in DMEM at 37 °C. At the indicated time points, cells were lysed, insoluble material was removed by centrifugation at 10000 g, and immune complexes were recovered with Protein A–Sepharose and analysed by SDS/PAGE. To compare the kinetics of N-terminus processing in wild-type cells and shedding-defective mutants that express inactive TACE, both cell types were labelled with 500 μCi/ml of [35S]cysteine for 20 min, and the label was chased in complete medium for various periods of time in DMEM at 37 °C. At the indicated time points, cells were lysed, and insoluble material was removed by centrifugation. HA–proTGFα was immunoprecipitated with anti-HA antibodies, and immune complexes were analysed as described previously [21].
Protease protection experiments
For protease protection experiments with proteinase K, CHOTGFα cells were plated in 60-mm-diameter dishes and were allowed to reach 90% confluence. Monolayers were then washed once with KRH buffer (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 6 mM glucose and 25 mM Hepes, pH 7.4) and incubated in 1 ml of this buffer supplemented with 200 μg/ml proteinase K for 30 min at room temperature (22 °C). After this time, efficient action of the protease was seen by full detachment of the cells from the dishes. Cells were then recovered in Eppendorf tubes, and pelleted to remove the proteinase K solution. After washing the cells three times with PBS containing 2 mM PMSF, cells were lysed in 1 ml of lysis buffer and analysed for TGFα fragment generation by using the anti-proTGFα antiserum and Western blotting.
Quantitative estimation of proTGFα forms
Quantification of the different proTGFα forms in Western blots was performed by using the NIH (National Institutes of Health) Image 1.61 software. To graphically represent the values of each band (15, 17 or 20 kDa), the intensity of each band was measured densitometrically. Then, the sum of the three bands (15+17+20 kDa) was taken as 100%. Next, the percentage intensity of each band with respect to the total of that sample was calculated and plotted. Unless otherwise indicated, the data show the means±S.D. for two different experiments.
RESULTS
Cell-associated molecular forms of proTGFα in CHOTGFα and HeLaTGFα cells
CHOTGFα cells have been used widely to analyse the stepwise synthesis, maturation and cleavage of proTGFα [10,17,36]. Pulse–chase experiments indicated that, in these cells, proTGFα is synthesized initially as a 17/18 kDa protein which is then chased into 20 and 22 kDa forms (Figure 1B). These bands correspond to forms of proTGFα that still retain the N-terminal region (Figure lA, and [10,17,36]). At later chase times, these bands disappear and are converted into a 17 kDa form, devoid of the N-terminal extension by cleavage at site 2. The 17 kDa form accumulates at the plasma membrane of CHOTGFα cells for over 2 h. This indicates that the 17 kDa form is very slowly cleaved under resting conditions (Figure 1B, and [10,17,36]).
To analyse the abundance of the different proTGFα forms under resting steady-state conditions, we performed Western blotting of proTGFα in CHOTGFα and HeLaTGFα cells using the C-terminal anti-proTGFα antibody (Figure 1C). In this situation, both CHOTGFα and HeLaTGFα cells accumulated forms of proTGFα of 20, 17 and 15 kDa. A diffuse autoradiographic signal between 20 and 22 kDa was also observed in CHOTGFα cells. The intensity of the 20–22 kDa region was lower than that of other proTGFα forms, and the detection by Western blotting of the 20 kDa and, especially, the 22 kDa forms was variable and difficult. Pre-incubation of the anti-proTGFα immunoprecipitates with an excess of the peptide against which the antiserum was raised, completely prevented the identification of the 22, 20, 17 and 15 kDa forms in CHOTGFα cells (Figure 1C). To analyse further the nature of the different proTGFα forms, we created antibodies against a GST-fusion protein that included the N-terminus of proTGFα and the TGFα domain (Figure 1D). Antibodies specific for each region were then purified by affinity chromatography using GST-fusion proteins that contained either the N-terminus or the TGFα module of proTGFα. The antibodies purified using GST–N-terminus recognized this protein in Western blots, but failed to react with GST–TGFα (Figure 1D). Conversely, the anti-TGFα antibody identified only the GST–TGFα fusion protein, but was unable to detect the GST–N-terminus fusion protein. Immunoprecipitation of CHOTGFα cell extracts with the anti-TGFα or with the anti-proTGFα antibodies, followed by Western blotting with the anti-TGFα (Figure 1E, right-hand panel) or with the anti-proTGFα (Figure 1E, left-hand panel) antibodies showed that the anti-TGFα antibody was able to recognize the 17 kDa form, and also reacted with the 20 and 22 kDa forms. However, this antibody failed to detect the 15 kDa band, indicating that this form had lost the TGFα domain within proTGFα. The anti-TGFα antibody also detected a 6 kDa band in the culture medium of CHOTGFα cells, but not in that of wild-type CHO cells (Figure 1F). This was the sole soluble TGFα band detected in the medium, as has been reported previously [17]. Under resting conditions, the amount of this band was discrete, but increased upon treatment with PMA, a drug previously reported to stimulate proTGFα cleavage with release of soluble TGFα into the culture medium [17]. Under these resting conditions, the anti-N-terminus antibody failed to clearly immunoprecipitate any of the proTGFα bands of CHOTGFα cells (see below, and Figure 2B).
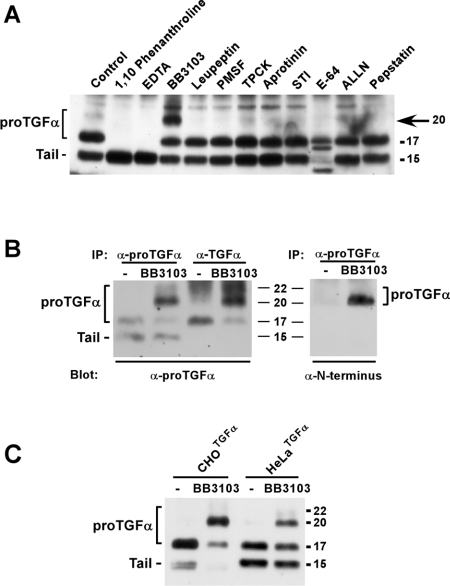
Figure 2. Effect of protease inhibitors on proTGFα forms.
(A) CHOTGFα cells were incubated for 4 h with 1,10-phenanthroline (1 mM), EDTA (5 mM), BB3103 (10 μM), leupeptin (10 μg/ml), PMSF (2 mM), tosylphenylalanylchloromethane (TPCK; 10 μM), aprotinin (10 μg/ml), soybean trypsin inhibitor (STI; 10 μg/ml), E64 (20 μM), N-acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN; 20 μM) or pepstatin (2 μM). Lysates were prepared and analysed by immunoprecipitation and Western blotting as described in the text. The arrow indicates the position of the 20 kDa form that accumulated upon BB3103 treatment. (B) Immunoprecipitation of the different proTGFα forms with the anti-proTGFα or the anti-TGFα antibodies in cells treated with BB3103. CHOTGFα cells were incubated overnight with BB3103 (10 μM), and then cell extracts were prepared and immunoprecipitated (IP) with the indicated antibodies. The Western blots were probed with anti-proTGFα antibodies (left-hand panel) or with anti-N-terminus antibodies (right-hand panel). (C) Action of BB3103 on the 20 and 22 kDa forms in CHOTGFα and HeLaTGFα cells. Where indicated, cells were pre-incubated overnight with 10 μM BB3103. Lysates were precipitated with the anti-proTGFα antibody, followed by Western blotting with the same antibody. Molecular masses are indicated in kDa in all panels.
The above data confirmed the presence of multiple cell-associated forms in CHOTGFα and HeLaTGFα cells, and indicated that the 20 and 22 kDa forms were short-lived in CHOTGFα cells, being converted into the more stable form of 17 kDa, devoid of the N-terminal extension.
Hydroxamic-acid-derived metalloprotease inhibitors induce accumulation of N-terminal-containing forms of proTGFα in vivo
The small amount of 22 and 20 kDa forms limited the study of certain properties of the shedding event that removes the N-terminal region. To overcome this, we attempted to pharmacologically prevent shedding of this N-terminal region to induce accumulation of the 20 and 22 kDa forms. To this end, we analysed the effect of several protease inhibitors on the levels of the 20 and 22 kDa forms. In vivo treatment of CHOTGFα cells with inhibitors of serine, cysteine and aspartic proteases were unable to produce accumulation of the 20 and 22 kDa forms (Figure 2A). The cysteine protease inhibitor E64 had a rather striking effect on the pattern of proTGFα forms, but since it did not clearly affect the 20 and 22 kDa forms, its action was not evaluated further. In contrast, the metalloprotease inhibitors significantly affected proTGFα cleavage. The hydroxamic-acid-derived inhibitor BB3103 increased the amount of the 20 kDa form of proTGFα, while decreasing the 17 kDa form (Figures 2A and 2B). The effect of BB3103 on the 22 kDa form was much less evident. That the 20 and 22 kDa bands corresponded to forms of proTGFα that included the N-terminal extension was verified by Western blotting with the anti-N-terminus antibody. In untreated CHOTGFα cells, the low amounts of the 20 and 22 kDa forms prevented their detection by the anti-N-terminus antibody (Figure 2B, right-hand panel). However, in the presence of BB3103, the antibody reacted with a band of 20 and 22 kDa. These data demonstrate that the forms that accumulate in the presence of BB3103 correspond to forms of proTGFα that include the N-terminal region of the precursor. Interestingly, EDTA and 1,10-phenanthroline had an unexpected effect, i.e. they caused a decrease of the 17 kDa form together with an increase of the 15 kDa cell-associated fragment. The action of BB3103 was reproduced in HeLaTGFα cells (Figure 2C), indicating that the machinery for N-terminal processing was also sensitive to this inhibitor not only in hamster, but also in human cells.
The effect of BB3103 on the N-terminal cleavage of proTGFα in vivo was found to be dose- and time-dependent. Inhibition was observed at concentrations as low as 1 μM, as seen by the accumulation of the 20 kDa form (Figure 3A, upper panel). The intensity of the 20 kDa band augmented progressively with increasing concentrations, while the amount of the 17 and 15 kDa bands decreased accordingly. BB3103 had a less evident effect on the 22 kDa form, which appeared as a faint band of much lower intensity than the 20 kDa band. It should be noted, however, that BB3103 caused accumulation in the diffuse zone that extended into the 20–22 kDa region.
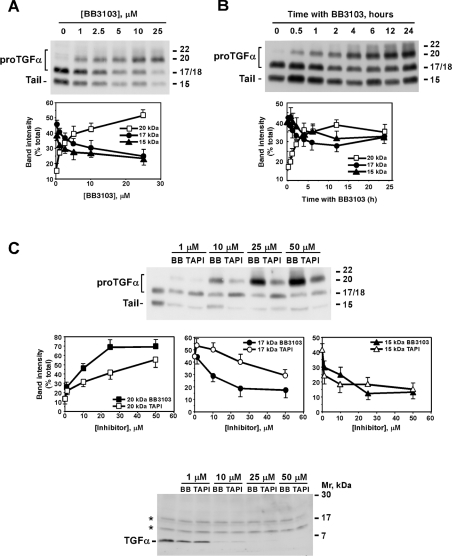
Figure 3. Time course and dose–response of hydroxamic-acid-derived inhibitors on the levels of the different proTGFα forms.
(A) Dose–response of the effect of BB3103. CHOTGFα cells were incubated overnight with the indicated concentrations of BB3103. The different proTGFα forms were analysed by Western blotting as described above. The graph shows the means±S.D. for two different experiments. (B) Time course of the effect of BB3103. CHOTGFα cells were incubated for the indicated times with 10 μM BB3103, and proTGFα was analysed by Western blotting. The graph shows the means±S.D. for two different experiments. (C) Dose–effect of BB3103 and TAPI-2 on the different proTGFα forms. CHOTGFα cells were incubated overnight with the indicated concentrations of BB3103 or TAPI-2, and the effect of these compounds on proTGFα (top panel) or soluble TGFα (bottom panel) was analysed by Western blotting. For the analysis of soluble TGFα, cells were placed in serum-free medium for 24 h in the absence or presence of the indicated concentrations of the inhibitors. Medium was concentrated, and TGFα was detected by Western blotting with the anti-TGFα antibodies. The asterisks (*) indicate two non-specific bands. The middle panels graphically represent the percentage intensity of each band (20 kDa, 17 kDa or 15 kDa) with respect to the total of each sample (15+17+20 kDa). For this measurement, the intensity of each individual band in a certain sample was calculated, and the sum was taken as 100%. Then, the percentage intensity of each band with respect to the total of that sample was calculated and plotted. Results are means±S.D. for two different experiments. Molecular masses are indicated in kDa for the gels.
Time-course experiments indicated that BB3103 had already induced accumulation of the 20 kDa band at 30 min of treatment with BB3103 (Figure 3B). The amount of the 20 kDa form increased progressively with time. This increase was rapid between 30 min and 4 h of treatment, and slower at 6 h and beyond (Figure 3B, lower panel). Concomitantly, the amount of the 17 and 15 kDa forms progressively decreased, except at 24 h of treatment, a time at which the intensity of both bands raised again. While this may be due to instability of BB3103 at those large time points, this appears to be unlikely, since the 20 kDa band was still present at these time points.
The action of TAPI-2, another hydroxamic-acid-derived inhibitor reported to affect membrane protein ectodomain cleavage [37], was also analysed and compared with the effect of BB3103. As shown in Figure 3(C), both compounds similarly affected 15 kDa accumulation along the range of concentrations evaluated. However, BB3103 preferentially increased the 20 kDa form with respect to the 17 kDa form at concentrations of 10, 25 and 50 μM (Figure 3C). TAPI-2 increased the amount of the 17 kDa to levels above those of the 20 kDa form, especially at 10 μM. At 25 μM TAPI-2, the proportion of the 20 and 17 kDa forms was almost identical, while at this concentration BB3103 caused a clear accumulation of the 20 kDa form, decreasing the 17 and 15 kDa forms (Figure 3C, top panel). We also analysed medium samples from cells treated with different concentrations of BB3103 or TAPI-2 to investigate whether these compounds caused accumulation of species of TGFα other than the 6 kDa form. As expected, increasing the concentrations of BB3103 or TAPI-2 progressively decreased accumulation of 6 kDa TGFα in the culture medium of CHOTGFα cells (Figure 3C, bottom panel). At 1 μM BB3103 or TAPI-2, a concentration that already caused inhibition of soluble TGFα accumulation in the culture medium, the only soluble TGFα form detected corresponded to the 6 kDa form.
N-terminal shedding of proTGFα occurs at the plasma membrane
The cellular site at which proTGFα N-terminal cleavage occurs has not been defined. We contemplated two possible cellular sites: the ER and the plasma membrane. The ER was considered, since cleavage at site 1 is expected to occur at this location, and N-terminal shedding happens shortly thereafter. On the other hand, the possibility that the N-terminal shedding occurred at the plasma membrane was based on the fact that cleavage of proTGFα at the C-terminus has been shown to occur at this location, and because the cleavage at the N- and C-terminal sites occurs between Ala–Val peptide bonds [12].
With the purpose of elucidating the cellular site where the machinery for the cleavage at the N-terminus was fully functional, we performed several types of experiments. We first investigated whether BB3103 could affect proTGFα retained in the ER. To this end, we treated CHOTGFα cells with brefeldin A, a compound known to trap proTGFα in the ER [12]. Brefeldin A accumulated a form of proTGFα that we name ≈20 kDa, since it migrated with a molecular mass very close to the 20 kDa form, but with slightly faster mobility (Figure 4A). Treatment with BB3103 was unable to change the mobility of the ≈20 kDa form in brefeldin A-treated cells, indicating that, when trapped intracellularly, the cleavage activities responsible for the shedding at site 2 are inactive or inaccessible to BB3103. In this experiment, we also used proteinase K to assess whether brefeldin A in fact caused entrapment of proTGFα in intracellular compartments. When CHOTGFα cells previously treated with brefeldin A were treated with proteinase K, the ≈20 kDa form was resistant to the action of proteinase K, indicating that in fact the ≈20 kDa form was inaccessible to the protease treatment. In contrast, in control CHOTGFα cells, the 17 kDa form was converted into a form of an apparent molecular mass of 15 kDa by proteinase K (Figure 4A). This form was indistinguishable, by SDS/PAGE, from the 15 kDa proTGFα tail present in untreated CHOTGFα cells, indicating that the 17 kDa form was exposed at the plasma membrane. In CHOTGFα cells treated with BB3103 to accumulate the 20 kDa form, treatment with proteinase K eliminated this form, indicating that the 20 kDa form may reach the plasma membrane. Further evidence for the latter was obtained by cell-surface immunoprecipitation experiments in CHO cells expressing an HA-tagged proTGFα. Cells were labelled for a short period of time with [35S]cysteine, and then chased for 30 min. Cells were then placed at 4 °C in the presence of the anti-HA antibody to react with cell-surface HA–proTGFα. After washing the antibody, cells were again incubated at 37 °C for the times indicated (Figure 4B). Under these conditions, the anti-HA antibody recognized the 20 kDa and the 17 kDa forms, indicating that both forms were exposed at the cell surface.
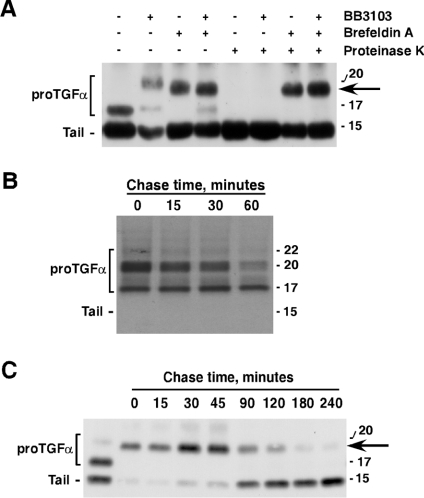
Figure 4. ProTGFα N-terminal shedding occurs outside the ER.
(A) Action of brefeldin A on proTGFα forms. CHOTGFα cells were incubated overnight with brefeldin A (10 μg/ml), and/or BB3103 (10 μM). Where indicated, proteinase K was added to the cells (200 μg/ml) for 30 min at room temperature. The different forms of proTGFα were analysed by Western blotting. The arrow indicates the position of the ≈20 kDa form. (B) CHO cells expressing HA-tagged proTGFα were labelled with 500 μCi/ml of [35S]cysteine for 20 min and then chased in complete medium for 30 min. Cells were then washed with cold PBS and incubated at 4 °C with 10 μg/ml of anti-HA for 1 h. Unbound anti-HA antibodies were washed, and the monolayers were incubated in DMEM at 37 °C for the indicated time points. Immune complexes were recovered and analysed by SDS/PAGE, followed by autoradiography. (C) CHOTGFα cells were incubated overnight with brefeldin A (10 μg/ml), and then chased from this compound for the indicated times. ProTGFα forms were analysed by Western blotting. Molecular masses are indicated in kDa in all panels.
We also analysed the recovery of N- and C-terminal cleavage after brefeldin-A-induced entrapment of proTGFα in the ER. In this experiment, CHOTGFα cells were treated with brefeldin A overnight, and the molecular forms of proTGFα were analysed by Western blotting at different times after release from brefeldin A. As shown in Figure 4(C), an increase in the ≈20 kDa form occurred at 30 min of chase, and this was accompanied by the generation of some 15 kDa forms. Increasing the chase time resulted in the decrease of the ≈20 kDa form together with increased 15 kDa levels, indicative of ≈20 into 15 kDa conversion. Interestingly, no 17 kDa was found during the time course of the chase, indicating that the ≈20 kDa conversion into the 15 kDa form occurred directly without the need for a previous step involving cleavage at site 2 to generate the 17 kDa form. We interpret these data as an indication of a more rapid recovery of the C-terminal shedding event, as compared with N-terminal shedding recovery.
The above data indicated that proTGFα N-terminal processing was inefficient in intracellular compartments, and showed that the 20 kDa form could accumulate at the plasma membrane. However, the data did not indicate whether the N-terminal processing machinery could act on 20 and 22 kDa forms already present at the plasma membrane. To address this, CHOTGFα cells were treated overnight with BB3103 to accumulate the 20 kDa form at the plasma membrane, and then chased for different times in the absence of the drug (Figure 5A). The amount of the 20 kDa band decreased over time, reaching steady-state values by 4 h of chase. The amount of the 17 and 15 kDa forms accumulated in parallel to the decrease in the 20 kDa form, indicating that the 20 kDa form was being converted into the 17 and 15 kDa forms.
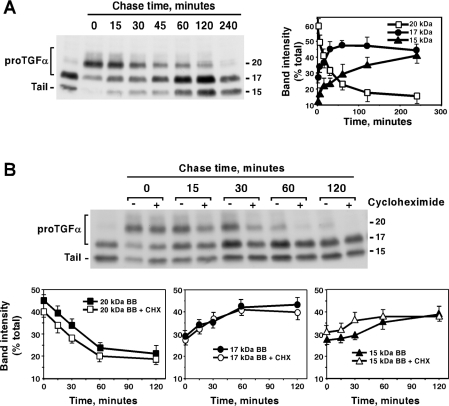
Figure 5. ProTGFα N-terminal shedding is reversible and may occur at the cell surface.
(A) CHOTGFα cells were incubated overnight with BB3103 (10 μM), and then chased for the indicated times in the absence of the compound. Cell lysates were prepared, and proTGFα forms were analysed by Western blotting (left-hand panel) and quantitative densitometry (right-hand panel). The right-hand panel shows the means±S.D. for two different experiments. (B) Action of CHX on the recovery of N-terminal shedding. CHOTGFα cells were incubated overnight with BB3103 (10 μM), and then chased for the indicated times in the absence of the drug. Where indicated, CHX (10 μg/ml) was added to the dishes 30 min before the start of the chase in BB3103-free medium, and CHX was kept throughout the experiment. Cell lysates were prepared, and proTGFα forms were analysed by Western blotting (upper panel) and quantitative densitometry (lower panels) as described in (A). The lower panels show the means±S.D. for two different experiments. Molecular masses are indicated in kDa for the blots.
These results suggested that the effect of BB3103 was reversible. However, the possibility that the reversal of the effect could be due to the synthesis of a new secretase was evaluated by performing an analogous experiment, but including CHX during the chase period. In this experiment, CHOTGFα cells were treated with BB3103 overnight, then 30 min before the start of the chase period, CHX was added to half of the dishes, and then cells were chased for the indicated times without BB3103, and with or without CHX, as indicated. As shown in Figure 5(B), addition of CHX did not prevent conversion of the 20 kDa into the 17 kDa form, indicating that protein synthesis was not required, and that therefore the reversible effect of BB3103 was due to termination of its inhibitory action on proteases already present. Even though, in CHOTGFα cells treated with CHX, the amount of the 22 kDa appeared to undergo a faster decrease than in untreated cells, quantitative analyses of the proportion of each band with respect to the others in each sample indicated that the kinetics of disappearance of the 20 kDa form were analogous in cells being treated or not with CHX (Figure 5B, lower panels). Therefore the apparent differences probably reflect decreases in proTGFα synthesis in CHOTGFα cells treated with CHX.
Secretases distinct from TACE process proTGFα efficiently at the N-terminus in vivo
To evaluate in vivo whether TACE was required for the processing of proTGFα at the N-terminus, we used two types of cells deficient in TACE activity: TACEΔZn/ΔZn cells [23] and the M2 CHO-derived mutant [34]. The former cell line was transfected with the rat cDNA coding for proTGFα, and the expression and maturation of proTGFα in several clones of TACEΔZn/ΔZn cells expressing different amounts of proTGFα was analysed by Western blotting (Figure 6A). Expression of proTGFα in TACEΔZn/ΔZn cells resulted in the accumulation of the 17 kDa form, as occurred in CHOTGFα cells (Figure 6A). However, and in contrast with the latter, very few 15 kDa forms were present under resting conditions, suggesting that TACE was required for 17 kDa into 15 kDa conversion, or that these cells cleaved proTGFα poorly at its C-terminus. Furthermore, treatment with activators of PKC (protein kinase C), such as PMA that causes cleavage of proTGFα in CHOTGFα and other cell types, did not affect cleavage of the 17 kDa form of proTGFα in TACEΔZn/ΔZn−TGFα cells (Figure 6B).
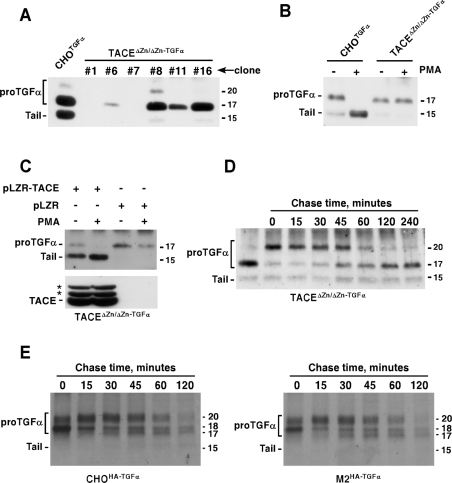
Figure 6. ProTGFα N-terminal shedding occurs in the absence of TACE activity.
(A) Expression of proTGFα in TACEΔZn/ΔZn cells. Cells were transfected with a vector coding for rat proTGFα, and clones selected. The expression of several clones with distinct levels of proTGFα is shown, compared with the expression in CHOTGFα cells. The immunoprecipitation and the Western blots were performed with the anti-proTGFα antibody. (B) Action of PKC activation by PMA on proTGFα cleavage. Where indicated, CHOTGFα or TACEΔZn/ΔZn−TGFα cells were treated with PMA (1 μM, 30 min), and the different proTGFα forms were analysed by Western blotting. (C) Reintroduction of TACE in TACEΔZn/ΔZn−TGFα cells rescues resting and PMA-induced cleavage. TACEΔZn/ΔZn−TGFα cells were infected with a retroviral vector containing TACE (pLZR-TACE), or with the empty retroviral vector (pLZR), and the expression of TACE was analysed by immunoprecipitation and Western blotting with an antibody that recognizes TACE (lower panel). The location of TACE is indicated, as well as two additional bands (asterisks) whose identity is unknown. The effect of reintroduced TACE on resting or PMA-induced (1 μM, 30 min) proTGFα cleavage was analysed by Western blotting (upper panel). (D) BB3103 causes reversible accumulation of N-terminal containing proTGFα forms in TACEΔZn/ΔZn−TGFα cells. TACEΔZn/ΔZn−TGFα cells were incubated overnight with 10 μM BB3103, and then chased in the absence of the drug for the indicated times. The different forms of proTGFα were analysed by Western blotting as above. (E) Wild-type or M2 mutant CHO cells expressing HA–proTGFα were labelled for 20 min with [35S]cysteine, and then chased for the indicated times in DMEM. Anti-HA immunoprecipitates were analysed by SDS/PAGE followed by autoradiography. Molecular masses are indicated in kDa in all panels.
TACE expression in TACEΔZn/ΔZn cells has been shown to increase the recovery of soluble TGFα in the culture media [22]. To assess the effect of TACE expression on the molecular forms of proTGFα expressed by TACEΔZn/ΔZn−TGFα cells, these cells were infected with a retrovirus that contained a vector that included the mouse wild-type TACE sequence. Infection resulted in the expression of TACE in these cells, as detected by Western blotting with anti-TACE antibodies (Figure 6C, lower panel). When TACE was reintroduced in TACEΔZn/ΔZn−TGFα cells, the 15 kDa form of proTGFα was already detectable under resting conditions. Treatment with PMA resulted in the cleavage of the 17 kDa form, that converted into the 15 kDa form (Figure 6C).
The lack of action of PMA on proTGFα cleavage in TACEΔZn/ΔZn−TGFα cells, but the presence of the 17 kDa protein suggested that TACE was required for PMA-induced cleavage at site 3, but that this metalloprotease could be dispensable for N-terminal processing of proTGFα in vivo. To analyse whether the 20 into 17 kDa conversion occurred analogously to that of CHOTGFα cells, TACEΔZn/ΔZn−TGFα cells were incubated with BB3103 overnight, and then chased at different time points in the absence of the metalloprotease inhibitor (Figure 6D). Incubation with BB3103 resulted in the accumulation of the 20 kDa band which, upon chase, was converted into the 17 kDa band with kinetics analogous to those observed in CHOTGFα cells (see also Figure 5A).
Finally, we also compared the kinetics of the 20 kDa form in CHO cells and the M2 mutant, both expressing HA-tagged proTGFα. The M2 cell line has been shown previously to bear a mutation in TACE that abolishes the activity of this protease [34]. Wild-type CHO cells and the M2 cells were briefly labelled with [35S]cysteine, and then chased for different periods of time. As shown in Figure 6(E), the kinetics of the 20 kDa form were analogous in both cell types. Taken together, these data indicate that the N-terminal extension of proTGFα was efficiently processed in the absence of TACE activity. However, TACE critically participated in PKC-induced proTGFα cleavage.
DISCUSSION
ProTGFα maturation and conversion into a soluble factor is a complex process that involves three proteolytic steps [3,9]. One, which occurs co-translationally, removes the N-terminal signal sequence. Another, occurring at the juxtamembrane domain, solubilizes TGFα, and the characteristics of this proteolytic step have been extensively studied [11]. However, the cleavage that removes the N-terminal extension of proTGFα has been poorly studied, mainly because of the rapid kinetics of this cleavage. In the present paper, we have designed a strategy that allowed us to answer several questions regarding this N-terminal cleavage, such as the cellular site where cleavage occurs, and the in vivo role of TACE as an N-terminal secretase.
The main purpose of our strategy was to accumulate in vivo proTGFα forms containing the N-terminal extension. It was also desirable that such accumulation would be reversible, to facilitate analysis of the kinetic properties of the N-terminal processing in vivo. We successfully accumulated proTGFα containing the N-terminus by using protease inhibitors of the hydroxamic acid family. This was expected, since the cleavage site of proTGFα at its N-terminus is coincident in primary sequence with the one that occurs at the C-terminus, and the latter is sensitive to protease inhibitors based on hydroxamates [21]. Furthermore, in vitro studies have indicated that hydroxamates may inhibit N-terminal cleavage [29]. However, the machinery for N-terminal cleavage could behave distinctly in vitro and in vivo. In fact, even though EDTA has been shown to efficiently prevent C-terminal processing in vitro [29], this compound had an opposite effect on proTGFα cleavage at this site in vivo. Thus addition of EDTA to CHOTGFα cells provoked processing at the C-terminus of proTGFα, causing accumulation of the 15 kDa form. Analogously, 1,10-phenanthroline, an unrelated metalloprotease inhibitor, also caused C-terminal processing of proTGFα. The basis for not only the failure of these metalloprotease inhibitors to prevent shedding at this site, but also the acceleration of the C-terminal shedding in vivo is unknown. However, phenotypically, these compounds caused detachment of CHOTGFα cells from the substrate. Reports from other groups have indicated that shedding can be regulated by adhesion [38]. In fact, in the case of heparin-binding EGF-like growth factor, lack of adhesion has been linked to resistance to proteolytic processing. It will be interesting to analyse whether adhesion represents a novel situation that regulates proTGFα cleavage.
In vivo treatment with BB3103 caused a time- and dose-dependent accumulation of the 20 and 22 kDa forms of proTGFα. However, the accumulation of the two forms was affected differentially. BB3103 caused a substantial accumulation of the 20 kDa form, and had a little, but reproducible, effect on the accumulation of the 22 kDa form. These data suggest that, while we could regulate the 20 kDa conversion into the 17 kDa by BB3103, the drug was not as effective in causing 22 kDa accumulation. This is interesting since pulse–chase experiments indicate that the proportion of both forms was analogous. However, the regulation of the amounts of the 22 kDa and 20 kDa appear to be different not only because of the distinct effect of BB3103, but also because of the Western blotting of steady-state proTGFα that indicated that the amount of the 22 kDa form was lower than the 20 kDa form, and was often difficult to detect. Clearly, more studies are required in order to elucidate the differences in behaviour between the 22 and the 20 kDa forms.
Comparative analysis of the inhibition profile by using TAPI-2 or BB3103 indicated differences between processing at the N- and C-terminus of proTGFα. Both agents similarly reduced the amount of the 15 kDa form, indicating that cleavage at the C-terminus of proTGFα was equally sensitive to both agents. At low concentrations, i.e. 1 and 10 μM, TAPI-2 caused a parallel increase in the 20 and 17 kDa forms, suggesting that both N- and C-terminal cleavages were equally sensitive to the action of this inhibitor. However, at these doses, BB3103 decreased the amount of the 17 kDa form while increasing the amount of the 20 kDa form. The distinct in vivo sensitivity suggests that the activities causing shedding at both sites may be different, with the one acting at the N-terminus being more sensitive to BB3103 than to TAPI-2. This conclusion falls in line with previous in vitro studies that indicated differences in the cleavage at both ends of proTGFα [29]. Interestingly, in the latter report, multiple soluble TGFα forms of 6, 15 and 29 kDa accumulated in the culture medium of R1 Ras-transformed rat liver cells. The presence of the 15 and 29 kDa forms was taken as an indication of TGFα forms cleaved at the C-terminus only. In our experimental model, however, we only detected 6 kDa soluble TGFα. This soluble form accumulated in the culture medium of CHOTGFα cells, and its amount decreased when cells were treated with hydroxamates. Our failure to detect 15 and 29 kDa forms in the culture medium may be due to a higher N-terminal processing efficiency of CHO cells compared with rat liver cells, or the fact that the proTGFα used in [29] was HA-tagged at the ectodomain at a position close to the N-terminal cleavage site.
The cellular site where N-terminal shedding occurred was unknown. Because of the rapid release of the N-terminus of proTGFα, the possibility that this shedding event occurred intracellularly shortly after proTGFα synthesis has been proposed [10]. Our results indicate that shedding at the N-terminus is likely to occur at the cell surface by a rapidly acting secretase and not intracellularly, at least not in the ER. This is interesting, since the protease responsible for the N-terminal shedding is expected to be produced together with proTGFα at this cellular site. The basis for the exclusion of the ER as a site for N-terminal shedding was supported by the experiments with brefeldin A. This drug has been reported to allow synthesis of proTGFα, but prevents its export from the ER [12]. Treatment with the drug caused accumulation of a form of proTGFα with a molecular mass analogous to that of the 20 kDa, together with some 15 kDa form, and almost no 17/18 kDa forms. The ≈20 kDa band in cells treated with brefeldin A was insensitive to proteinase K, indicating that this form was in fact trapped intracellularly and was not exposed at the plasma membrane. In addition, the ≈20 kDa form was insensitive to BB3103, indicating either that the drug would not be able to access intracellularly or that indeed the ≈20 kDa intracellularly trapped form was insensitive to the N-terminal processing. This appears to be the more likely possibility, since no generation of the 17 kDa form was detected in cells treated with brefeldin A. An interesting conclusion can also be obtained from the data of cells treated with brefeldin A and then chased in the absence of this compound. Surprisingly, the ≈20 kDa form was converted into the 15 kDa form without previous generation of the 17 kDa form. This indicates that the C-terminal cleavage is more efficiently recovered than the N-terminal cleavage, and contrasts with the rapid recovery of N-terminal shedding upon elimination of BB3103. This finding adds further evidence supporting that the shedding machineries acting at both cleavage sites are distinct, or at least distinctly regulated.
Several direct and indirect findings have pointed to the plasma membrane as the site where the machinery for N-terminal shedding is fully active. Thus both the N- and C-terminal cleavage sites occur between Ala–Val peptide bonds, and the C-terminal cleavage has been shown to occur at the cell surface [12]. More direct evidence has been obtained by our experiments using BB3103. In fact, treatment with BB3103 caused an increase in cell-surface 20 kDa proTGFα, as indicated by its sensitivity to proteinase K. Since BB3103 is expected to act on surface-exposed metalloproteases, the accumulation of the 20 kDa form was indicative that the BB3103-sensitive protease was located at the plasma membrane. In addition, cell-surface immunoprecipitation experiments indicated that both the 20 kDa and the 17 kDa forms were present at the cell surface. These data, together with the fact that, upon accumulation of the 20 kDa form at the plasma membrane by BB3103, it could be converted into the 17 kDa form, indicate that both the substrate and the processing enzyme(s) reside at the plasma membrane. Furthermore, the rapid conversion of the 20 kDa form into the 17 kDa form together with the in-sensitivity of the N-terminal shedding to CHX indicates that recovery of the activity is reversible, and does not depend on the neosynthesis of the shedding machinery involved.
With regard to the secretase implicated in the N-terminal shedding of proTGFα, our studies indicated that TACE is not essential. This is of particular interest since this protease has been shown to cleave the N-terminal site in vitro with high efficiency, and has been proposed as the major proTGFα N-terminal secretase [22,29]. However, our in vivo results using TACEΔZn/ΔZn−TGFα and M2 cells indicated that TACE, while being very important for resting and PKC-mediated shedding of proTGFα, it is dispensable for the shedding at the N-terminus. Thus Western blotting of proTGFα in TACEΔZn/ΔZn−TGFα cells under steady-state conditions indicated that the major proTGFα form corresponded to the 17 kDa form. This first finding showed that TACE was dispensable for the N-terminal processing, because if TACE was required, we would expect accumulation of the 20 and 22 kDa forms. Furthermore, treatment with BB3103 accumulated the 20 kDa form, and this accumulation could be reversed following a time course similar to that of CHOTGFα cells, upon elimination of BB3103. In addition, in M2 cells that express a mutated inactive form of TACE [34], proTGFα maturation was indistinguishable from that of wild-type CHO cells. Therefore, even though TACE appears to be an efficient in vitro converting protease for the N-terminal shedding, our data in intact cell lines indicate that other proteases may efficiently cleave off the N-terminus. Furthermore, our data not only indicate that TACE is dispensable for N-terminal processing, but also question whether, in intact cells, TACE is required at all for this processing. This represents an important point that does not challenge the former in vitro data, but rather stresses the importance of complementing in vitro and in vivo studies to better understand the characteristics of proTGFα maturation and processing.
An important question that remains to be addressed refers to the identification of the molecular machinery required for the N-terminal maturation and processing. In addition to TACE, ADAM10 has also been proposed to be able to cleave proTGFα at the N-terminus [22,29]. Of the 39 ADAMs known, we have identified by reverse transcriptase PCR five potential candidates, ADAMs 9, 10, 12, 15 and 19, that may act as N-terminal sheddases (P. P. Juanes and A. Pandiella, unpublished work). Experiments of proTGFα expression in cells from single and multiple knockout of these ADAMs, when available, will define the importance of these proteases in proTGFα N-terminal shedding.
Acknowledgments
We thank the scientists and companies (Amgen-Immunex and British Biotech) mentioned in the Experimental section for the gifts of reagents. This work was supported by a grant from the FISS (01/1060) and from the Ministry of Science and Technology of Spain (BMC2003-01192). P.P.J., L.F. and J.C.M. were supported by the ISCIII-Ministry of Health.
References
- 1.DeLarco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. U.S.A. 1978;75:4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derynck R. The physiology of transforming growth factor-α. Adv. Cancer Res. 1992;58:27–52. doi: 10.1016/s0065-230x(08)60289-4. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J. Transforming growth factor-α: a model for membrane-anchored growth factors. J. Biol. Chem. 1990;265:21393–21396. [PubMed] [Google Scholar]
- 4.Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF-α in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 5.Matsui Y., Halter S. A., Holt J. T., Hogan B. L. M., Coffey R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF α transgenic mice. Cell. 1990;61:1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 6.Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF-α overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 7.Teixidó J., Gilmore R., Lee D. C., Massagué J. Integral membrane glycoprotein properties of the prohormone pro-transforming growth factor-α. Nature (London) 1987;326:883–885. doi: 10.1038/326883a0. [DOI] [PubMed] [Google Scholar]
- 8.Bringman T. S., Lindquist P. B., Derynck R. Different transforming growth factor-α species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987;48:429–440. doi: 10.1016/0092-8674(87)90194-2. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J., Pandiella A. Membrane-anchored growth factors. Annu. Rev. Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 10.Teixidó J., Wong S. T., Lee D. C., Massagué J. Generation of transforming growth factor-α from the cell surface by an O-glycosylation-independent multistep process. J. Biol. Chem. 1990;265:6410–6415. [PubMed] [Google Scholar]
- 11.Harris R. C., Chung E., Coffey R. J. EGF receptor ligands. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 12.Bosenberg M. W., Pandiella A., Massagué J. The cytoplasmic carboxy-terminal amino acid specifies cleavage of membrane TGF-α into soluble growth factor. Cell. 1992;71:1157–1165. doi: 10.1016/s0092-8674(05)80064-9. [DOI] [PubMed] [Google Scholar]
- 13.Briley G. P., Hissong M. A., Chiu M. L., Lee D. C. The carboxyl-terminal valine residues of proTGFα are required for its efficient maturation and intracellular routing. Mol. Biol. Cell. 1997;8:1619–1631. doi: 10.1091/mbc.8.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentry L. E., Twardzik D. T., Jonathan Lin G., Ranchalis J. E., Lee D. C. Expression and characterization of transforming growth factor precursor α protein in transfected mammalian cells. Mol. Cell. Biol. 1987;7:1585–1591. doi: 10.1128/mcb.7.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier N., Derynck R. Transmembrane TGF-α precursors activate EGF/TGF-α receptors. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 16.Atlas E., Cardillo M., Mehmi I., Zahedkargaran H., Tang C., Lupu R. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol. Cancer Res. 2003;1:165–175. [PubMed] [Google Scholar]
- 17.Pandiella A., Massagué J. Cleavage of the membrane precursor for transforming growth factor α is a regulated process. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1726–1730. doi: 10.1073/pnas.88.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandiella A., Massagué J. Multiple signals activate cleavage of membrane transforming growth factor-α precursor. J. Biol. Chem. 1991;266:5769–5773. [PubMed] [Google Scholar]
- 19.Fan H., Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosenberg M. W., Pandiella A., Massagué J. Activated release of membrane-anchored TGF-α in the absence of cytosol. J. Cell Biol. 1993;122:95–101. doi: 10.1083/jcb.122.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arribas J., Coodly L., Vollmer P., Kishimoto T. K., Rose-John S., Massagué J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- 22.Sunnarborg S. W., Hinkle C. L., Stevenson M., Russell W. E., Raska C. S., Peschon J. J., Castner B. J., Gerhart M. J., Paxton R. J., Black R. A., Lee D. C. Tumor necrosis factor-α converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 23.Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 24.Black R. A., White J. M. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 1998;10:654–659. doi: 10.1016/s0955-0674(98)80042-2. [DOI] [PubMed] [Google Scholar]
- 25.Merlos-Suarez A., Ruiz-Paz S., Baselga J., Arribas J. Metalloprotease-dependent protransforming growth factor-α ectodomain shedding in the absence of tumor necrosis factor-α-converting enzyme. J. Biol. Chem. 2001;276:48510–48517. doi: 10.1074/jbc.M103488200. [DOI] [PubMed] [Google Scholar]
- 26.Teixidó J., Massagué J. Structural properties of a soluble bioactive precursor for transforming growth factor-α. J. Biol. Chem. 1988;263:3924–3929. [PubMed] [Google Scholar]
- 27.Luetteke N. C., Michalopoulos G. K., Teixidó J., Gilmore R., Massagué J., Lee D. C. Characterization of high molecular weight transforming growth factor α produced by rat hepatocellular carcinoma cells. Biochemistry. 1988;27:6487–6494. doi: 10.1021/bi00417a043. [DOI] [PubMed] [Google Scholar]
- 28.Ignotz R. A., Kelly B., Davis R. J., Massagué J. Biologically active precursor for transforming growth factor type α, released by retrovirally transformed cells. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6307–6311. doi: 10.1073/pnas.83.17.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinkle C. L., Mohan M. J., Lin P., Yeung N., Rasmussen F., Milla M. E., Moss M. L. Multiple metalloproteinases process protransforming growth factor-α (proTGF-α) Biochemistry. 2003;42:2127–2136. doi: 10.1021/bi026709v. [DOI] [PubMed] [Google Scholar]
- 30.Schlondorff J., Becherer J. D., Blobel C. P. Intracellular maturation and localization of the tumour necrosis factor α convertase (TACE) Biochem. J. 2000;347:131–138. [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Rodriguez E., Montero J. C., Esparis-Ogando A., Yuste L., Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor α-converting enzyme at threonine 735: a potential role in regulated shedding. Mol. Biol. Cell. 2002;13:2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1987. [Google Scholar]
- 33.Pandiella A., Bosenberg M. W., Huang E. J., Besmer P., Massagué J. Cleavage of membrane-anchored growth factors involves distinct proteolytic activities regulated through common mechanisms. J. Biol. Chem. 1992;267:24028–24033. [PubMed] [Google Scholar]
- 34.Villanueva de la Torre T., Bech-Serra J. J., Ruiz-Paz S., Baselga J., Arribas J. Inactivating mutations block the tumor necrosis factor-α-converting enzyme in the early secretory pathway. Biochem. Biophys. Res. Commun. 2004;314:1028–1035. doi: 10.1016/j.bbrc.2003.12.186. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera N., Díaz-Rodríguez E., Becker E., Zanca D. M., Pandiella A. TrkA receptor ectodomain cleavage generates a tyrosine-phosphorylated cell-associated fragment. J. Cell Biol. 1996;132:427–436. doi: 10.1083/jcb.132.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baselga J., Mendelsohn J., Kim Y. M., Pandiella A. Autocrine regulation of membrane transforming growth factor-α cleavage. J. Biol. Chem. 1996;271:3279–3284. doi: 10.1074/jbc.271.6.3279. [DOI] [PubMed] [Google Scholar]
- 37.Mullberg J., Rauch C. T., Wolfson M. F., Castner B., Fitzner J. N., Otten-Evans C., Mohler K. M., Cosman D., Black R. A. Further evidence for a common mechanism for shedding of cell surface proteins. FEBS Lett. 1997;401:235–238. doi: 10.1016/s0014-5793(96)01480-9. [DOI] [PubMed] [Google Scholar]
- 38.Gechtman Z., Alonso J. L., Raab G., Ingber D. E., Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J. Biol. Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]