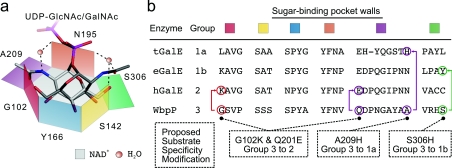
Figure 1. Model of the substrate-binding site of WbpP and other C4 epimerases.
(a) The substrate-binding pocket is represented by an hexagon. Three of the six sides are made of the three conserved Ser142 (yellow), Tyr166 (blue) and Asn195 (orange) residues in WbpP. The three other sides are made of Gly102 (red), Ala209 (purple) and Ser306 (green). (b) The corresponding residues found in other homologues are indicated, and a selection of mutations tested in the present paper is highlighted. The UDP-GlcNAc and UDP-GalNAc bound in catalytically productive conformations are represented by a solid line. The role of water molecules is signified by spheres. Adapted from [4] with permission. © 2004 American Society for Biochemistry and Molecular Biology.