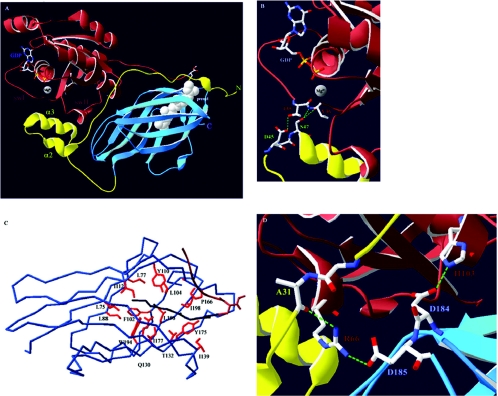
Figure 1. Cdc42–RhoGDIα interactions.
(A) Overview of the Cdc42–RhoGDIα complex. Cdc42 is shown in brown and its isoprenyl moiety in white space-fill (prenyl). The nucleotide (GDP) and the Mg2+ ion, as well as the switch I and II regions (swI and swII respectively), of Cdc42 are also indicated. The N-terminal domain of RhoGDIα is in yellow with the helix–loop–helix motif indicated through helices α2 and α3. The C-terminal immunoglobulin-like motif is in blue. (B) Nucleotide binding occurs through co-ordination of the Mg2+ ion by the main chain carbonyl group of Thr35(Cdc42). RhoGDIα stabilizes the GDP-bound form and prevents nucleotide dissociation through hydrogen bonds (dashed green lines) occurring between the carboxy oxygen of Asp45(RhoGDI) and the hydroxy group of Thr35(Cdc42) and between the hydroxy group of Ser47(RhoGDI) and the main chain amide and carbonyl groups of Val36(Cdc42). (C) Overview of the hydrophobic pocket of RhoGDIα. Shown is the α–carbon atom trace. Conserved hydrophobic amino acids (red) that form favourable van der Waals contacts along the length of the geranylgeranyl group (dark blue) line the pocket. Also indicated is the C-terminal hypervariable region of Cdc42 (brown). (D) Arg66(Cdc42) constitutes an important residue for the interaction with RhoGDIα as it can interact with amino acids from both the N- and C-terminal domains of RhoGDIα. The hydrogen bonds between the side chain of Arg66(Cdc42) and the main chain carbonyl group of Ala31(RhoGDI) and the side chain of Asp185(RhoGDI) are indicated. Also shown is the highly conserved interaction between Asp184(RhoGDI) and His103(Cdc42). Mutation of either of these residues on GTPases results in RhoGDI-binding deficiency [58–60,62,66].